Introduction
Lung cancer exhibits alarmingly high incidence and mortality rates both globally and in the Republic of Moldova. In 2021, an estimated 2 million cases occurred worldwide, with 1.8 million deaths. The American Cancer Society projects 236.740 new cases and 130.180 deaths in the US for 2022 alone. Lung cancer tragically accounts for almost 20% of all cancer-related deaths [1-3].
Surgical intervention remains pivotal in diagnosing, staging, and treating non-small cell lung cancer (NSCLC). Lung resection is the gold standard approach for stage I-II NSCLC and a vital part of multimodal treatment in stage IIIA [4]. Standard resections (lobar, bilobar, pulmonary) with ipsilateral hilar and mediastinal lymph node revision are common practices. For early-stage cancers, reducing morbidity and mortality is the primary surgical objective [5]. In advanced stages or patients facing heightened surgical risks, careful selection is necessary to ensure that those most likely to benefit from surgery, potentially combined with radiotherapy or chemotherapy, are prioritized [6, 7].
A burgeoning trend in perioperative management is prehabilitation (respiratory, cognitive, motor, etc.). This multifaceted approach aims to enhance a patient's functional reserve, better equipping them to withstand surgical stress and optimize postoperative recovery. Additionally, the impact of techniques like fascial plane blocks and antifibrinolytics on thoracic cancer surgery outcomes remains understudied [8, 9].
Material and methods
This investigation employed a two-phased, observational cohort design: retrospective and prospective. The study protocol received approval from the Research Ethics Committee of Nicolae Testemiţanu State University of Medicine and Pharmacy, Republic of Moldova (Protocol No. 04 of 12.11.2020). Written informed consent was obtained from all prospectively enrolled patients.
To achieve study objectives, two patient cohorts were established. The retrospective cohort (100 patients) was derived from the Oncology Institute database. Patients with lung cancer and documented high anesthetic risk were included if they met pre-defined enrollment criteria and were not offered surgery due to traditional contraindications. The prospective cohort (100 patients) included patients meeting the same enrollment criteria who underwent surgery based on the newly proposed approach. This approach incorporated prehabilitation, accelerated postoperative rehabilitation, anterior serratus fascial plane block, intraoperative antifibrinolytic therapy, and risk stratification using integrated scores (ASA, Th-RCRI, MET, Charlson).
Within the operated group, a sub-analysis was conducted to identify differences, risk factors, and predictors of postoperative mortality. Survivors were compared to early postoperative death. Finally, the long-term outcome (2-year survival rate) of surgically treated patients (under extended criteria) was compared to the historical cohort receiving only chemotherapy and/or radiotherapy (traditional approach). Descriptive and inferential statistical analyses were performed to evaluate the collected data. The results were subsequently utilized to develop a mathematical model for risk stratification. This model, based on identified and quantified risk factors (parameterized), aimed to refine surgical indications and contraindications. Additionally, it informed the formulation of general practice recommendations.
Two patient subcategories within the high anesthetic-surgical risk group emerged during analysis. These subgroups demonstrated comparable outcomes: (1) Stage III lung cancer patients with a Charlson score of 0-4 points; (2) Stage I-II lung cancer patients with a Charlson score of 5-12 points.
G*Power v. 3.1.9.6 (Franz Faul, University of Kiel) was used to determine the minimum sample size needed to detect a clinically meaningful difference of at least 15% in the two-year survival rate between groups. This calculation yielded a target enrollment of at least 184 patients. Data analysis was performed using GraphPad Prism software (version 9 trial). Statistical tests were chosen based on the specific research hypothesis, data distribution characteristics, and the number of data series involved. These included: (1) Parametric: t-Student test; (2) Non-parametric: Fisher exact test, Spearman/Pearson correlations, Mann-Whitney U test; (3) Survival Analysis: Kaplan-Meier curves with Mantel-Cox test; (4) Modeling: Logarithmic regression, probability calculations, multicollinearity testing.
Results are presented as mean ± standard deviation (with 95% confidence intervals where applicable) or relative frequencies. A p-value of <0.05 was considered statistically significant, with a study power of 80%, alpha error of 5%, and beta error of 20%.
Results
Given the limited cure rates associated with lung cancer, regardless of stage, therapeutic strategies primarily focus on extending life expectancy. Therefore, two critical outcome measures were used: (1) Life expectancy (in months): Measured from the date of diagnosis; (2) Survival rates at specific intervals: Commonly assessed at the 5-year mark. Results from patients receiving traditional therapy (the reference group) were analyzed using various covariates: (1) Stage of disease at diagnosis: A critical determinant of prognosis; (2) Charlson comorbidity index: Quantifies the burden of comorbid conditions; (3) Concurrent lung pathologies: Examines their impact on outcomes; (4) Treatment modalities utilized: Evaluates the effectiveness of existing therapies. Survival rates derived from this analysis will be compared to established literature data to assess the performance of the traditional management approach.
The frequency distribution highlights a stark reality: 40% survival at 1 year, 32.8% at 1.5 years, and only 8.2% at 2 years. Survival rates beyond 2 years remained at 8.0% (16 / 200 patients), with a mere 1% (2 / 200) reaching the 5-year mark. The mean survival time was 1.30±0.82 years. These findings underscore the urgent need for treatment innovations; even a 0.5-year increase in mean survival would be considered significant progress. Analyzing potentially modifiable factors (covariates) that influence survival may hold the key to such improvements. Key areas of exploration include the impact of comorbidities, concurrent lung pathologies, and the potential for optimizing existing treatment strategies (Table 1).
Table 1. Survival time, by disease stage and burden of comorbidities, expressed by the Charlson Comorbidity Index (CCI). | ||||
Duration between 'year of registration' and 'year of death', years | ||||
Stage I n=2 / 200 (1%) | Stage II n=10 / 200 (5%) | Stage III n=51 / 200 (25.5%) | Stage IV n=137 / 200 (68.5%) | |
Charlson 0 points | - | - | 0.8 (0.4 – 1.2) | 0.9 (0.7 – 1.1) |
Charlson 1-2 points | - | 1.3 (0.6 – 2.1) | 1.2 (0.9 – 1.5) | 1.4 (1.1 – 1.6) |
Charlson 3-4 points | 1.7 (-7.7 – 11.2) | 2.5 (-0.2 – 5.2) | 1.2 (0.8 – 1.5) | 1.4 (1.2 – 1.6) |
Charlson 5≤ points | - | 1.5* | 1.6 (0.8 – 2.3) | 1.2 (0.8 – 1.5) |
Note: Data expressed in years, mean, and 95% confidence interval (95% CI). Defaults indicate the lack of patients with the given characteristics in the study group (certain stage versus CCI score). *- only one patient in the group with the given characteristics. “Stage IV" column - patients ineligible for surgical treatment. Grey box - patients with lung cancer and increased anesthetic/surgical risk. | ||||
Given the disproportionate number of patients diagnosed with stage III and IV disease (94%), survival analysis primarily reflects outcomes for advanced stages. While stage I and II data represent individual cases, a focus on stages III and IV offers greater statistical relevance. Despite analyzing advanced stages, no significant differences in survival emerge, with mean survival ranging from 0.8 to 1.6 years. This emphasizes the poor prognosis associated with late-stage lung cancer and the urgent need for therapeutic advances across all stages.
Due to the small number of stage I and II cases, survival analysis from diagnosis to treatment initiation (radiotherapy or chemotherapy) is primarily descriptive for these early stages. Regarding advanced stages, neither chemotherapy nor radiotherapy alone appears to significantly impact survival. However, combining these modalities may yield an average survival gain of 6-8 months as presented (Table 2). These findings suggest a potential benefit of combination therapy and highlight the need for further investigation with larger sample sizes across all disease stages.
Table 2. Survival time, by stage of disease and by treatment (radiotherapy, chemotherapy, alone or in combination). | ||||
Treatment | Duration between 'year of registration' and 'year of death', years | |||
Stage I n=2 / 200 (1%) | Stage II n=10 / 200 (5%) | Stage III n=51 / 200 (25.5%) | Stage IV n=137 / 200 (68.5%) | |
Isolated radiation therapy | 2.5* | 2.0* | - | 2.0* |
Isolated chemotherapy | - | - | 0.9 (0.6 – 1.2) | 1.4 (1.1 – 1.6) |
Radio + chemotherapy | - | - | 1.6 (0.9 – 2.3) | 2.2 (1.1 – 3.3) |
Note: Data expressed in years, mean, and 95% confidence interval (95% CI). Defaults indicate lack of patients with the given characteristics in the study group (certain stage vs. treatment). *- only one patient in the group with the given characteristics. Column "stage IV" - patients not eligible for surgical treatment. | ||||
Table 3. Survival time, by stage of disease and by treatment (radiotherapy, chemotherapy, alone or in combination). | ||||
Symptom | Duration between 'year of registration' and 'year of death', years | |||
Stage I n=2 / 200 (1%) | Stage II n=10 / 200 (5%) | Stage III n=51 / 200 (25.5%) | Stage IV n=137 / 200 (68.5%) | |
Fatigue | 1.7 (-7.7 – 11.2) | 1.8 (0.7 – 2.9) | 1.2 (0.9 – 1.4) | 1.2 (1.1 – 1.3) |
Hemoptysis | 1.0* | 1.1 (0.4 – 1.8) | 1.2 (0.9 – 1.4) | 1.2 (1.0 – 1.4) |
Chest pain | - | 1.2 (-14.6 – 17.1) | 1.1 (0.8 – 1.3) | 1.3 (1.1 – 1.5) |
Dry cough | - | 2.0 (0.9 – 3.0) | 1.3 (1.0 – 1.5) | 1.3 (1.1 – 1.4) |
Weight loss | 1.7 (-7.7 – 11.2) | 1.8 (0.7 – 2.9) | 1.2 (1.0 – 1.4) | 1.2 (1.1 – 1.3) |
Dyspnea | 1.7 (-7.7 – 11.2) | 1.9 (0.8 – 3.0) | 1.2 (0.9 – 1.4) | 1.3 (1.1 – 1.4) |
Note: Data expressed in years, mean, and 95% confidence interval (95% CI). Defaults indicate lack of patients with the given characteristics in the study group (certain stage vs. treatment). *- only one patient in the group with the given characteristics. Column "stage IV" - patients not eligible for surgical treatment. | ||||
Certain lung cancer symptoms, while nonspecific, reflect the severity of underlying disease processes. These symptoms may indicate inflammation, metabolic disruptions, or consequences of tumor growth (invasion, compression). Advanced disease often necessitates complex compensatory mechanisms, potentially reducing patient resilience. We hypothesized that specific symptom profiles could be associated with shorter survival times. To investigate this, we analyzed data from the 200 lung cancer patients in our reference group (Table 3).
Our analysis of symptom presentation and survival (Table 3) revealed no statistically significant differences in survival time across various symptoms. Mean survival ranged from 1.1 to 2.0 years, with the majority of patients surviving 1.2-1.3 years on average. These findings suggest that symptom-based survival prediction in this context may be limited. This could be due to several factors: (1) Symptom Concurrence: Patients often experience multiple concurrent symptoms at different disease stages; (2) Tumor Heterogeneity: Tumor location and morphology can vary considerably. Therefore, further development of symptom-based prediction models in this specific patient population may be less promising. However, these results do not preclude the potential utility of symptom analysis in other contexts or alongside other prognostic factors.
We also investigated the relationship between various lung pathologies concurrent with lung cancer diagnosis (pleural effusion, pneumonia, pneumofibrosis, emphysema, spontaneous pneumothorax, hydrothorax, endobronchitis, and atelectasis) and survival time (Table 4). Our focus was to determine if specific associated pathologies could influence patient outcomes and potentially guide treatment decisions.
Table 4. Survival time, by stage of disease and lung pathological conditions, associated with lung cancer. | ||||
Parameters | Duration between 'year of registration' and 'year of death', years | |||
Stage I n=2 / 200 (1%) | Stage II n=10 / 200 (5%) | Stage III n=51 / 200 (25.5%) | Stage IV n=137 / 200 (68.5%) | |
Pleural effusion | - | - | 1.1 (0.07 – 1.2) | 1.3 (1.0 – 1.5) |
Pneumonia | - | 1.2 (-1.9 – 4.4) | 1.3 (0.7 – 1.9) | 1.3 (0.7 – 1.9) |
Pneumofibrosis | 2.5* | 2.3 (-0.4 – 5.2) | 1.5 (0.2 – 2.7) | 1.3 (0.9 – 1.7) |
Emphizema | - | - | 3.0* | 0.8 (0.2 – 1.3) |
Pneumothorax | - | - | 1.0* | 1.5 (-4.8 – 7.8) |
Hidrothorax | - | - | - | 0.7 (-2.4 – 3.9) |
Endobronchitis | - | 2.0* | 1.4 (0.8 – 1.9) | 1.5 (1.2 – 1.8) |
Lung colaps | - | 1.0* | 1.1 (0.5 – 1.6) | 1.3 (0.9 – 1.8) |
Note: Data expressed in years, mean, and 95% confidence interval (95% CI). Defaults indicate lack of patients with the given characteristics in the study group (certain stage vs. lung pathological conditions). *- only one patient in the group with the given characteristics. Column "stage IV” - patients ineligible for surgical treatment. | ||||
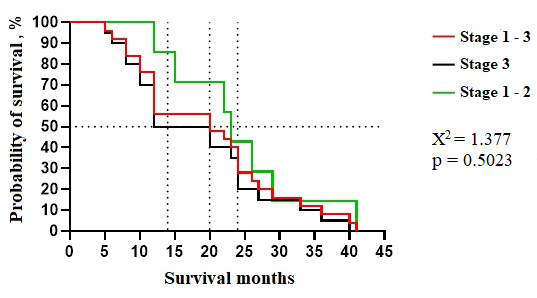
Analysis revealed that most associated lung pathologies manifest primarily in stage III lung cancer, becoming increasingly prevalent in terminal stages. While stages I-II exhibit occasional or limited occurrences of these conditions, survival times within this group show greater variability. Despite this, statistical analysis found no significant survival differences linked to specific pathologies. Median survival times ranged from 0.8 to 1.5 years, with a mode of 1.3 years. Our reference group comprised 200 lung cancer patients representing diverse disease stages at diagnosis. This group exhibited a range of comorbidities and associated lung pathologies. We conducted a multi-faceted analysis of these factors to establish comprehensive baseline group characteristics. The prospective study arm enrolled 86 patients who underwent lung cancer surgery following our innovative perioperative management protocol. We focused on two primary endpoints: survival time after diagnosis and the two-year survival rate. These illustrate potential outcomes for patients who would have been theoretically eligible for surgery under our protocol. For comparison, Figure 1 also includes outcomes from a subset of our reference group meeting specific criteria: age 38-75, stage I-III disease, and a Charlson Comorbidity Index (CCI) below 12.
|
Fig. 1 Kaplan-Mayer survival duration curve of patients with lung cancer (stage 1-3), excluded SCLC, without severe comorbidities (ICC over 12 points), with an age limit of 38-75 years, from the reference group (patients treated without surgery). Note: Χ2 - for linear trends (extended Hantel-Haenszel); p - survival analysis by Kaplan-Meier curves and log-rank (Mantel-Cox) test |
Mantel-Cox analysis of Kaplan-Meier curves revealed no statistically significant differences in survival times for patients theoretically eligible for surgery under our innovative approach. Within this subgroup (combining stages I-III), median survival was 20 months, and the two-year survival rate was 38.2% (13/34). One patient achieved an exceptional survival time of 41 months (~3.4 years). Stratifying by disease stage, we observed a median survival of 12 months and a two-year survival rate of 35.0% (7/20) for stage III patients. The mean survival was 18.3 months, with a range of 5 to 40 months (~0.4 - 3.3 years). For patients with stage I-II disease, median survival was 14 months, and the two-year survival rate was 42.9% (3/7), with a mean survival of 17.6 months and a range of 12 to 41 months (~1.0 - 3.4 years).
Discussions
Rjabov A et al. demonstrated the safety and efficacy of surgical treatment in selected lung cancer patients over 75 years of age. Key risk factors for postoperative complications included stage IIIb disease, lymph node involvement, and central cancers [10]. In this study of 73 patients, lobectomy was the most common procedure, with lymph node metastases noted in 32.9% of cases. Multivariate analysis identified the following significant risk factors: stage IIIb (OR=9.3, 95%CI=1.365-63.816; p=0.023), pN1 (OR=3.889, 95%CI=1.008-14.999; p=0.049), pN2 (OR=5.9, 95% CI=1.170-23.999; p=0.030), and central cancer (OR=7.572, 95%CI=1.742-32.884; p=0.007). Advances in diagnosis and treatment have significantly improved survival rates in NSCLC, which represents 80% of lung cancer cases [11].
While immunotherapy and targeted pharmacotherapy have significantly improved patient prognosis, the optimal treatment for advanced NSCLC remains an active area of research. Although surgery is generally not recommended for advanced NSCLC, particularly in cases of distant metastasis, some studies suggest potential benefits in selected patients with stage IV disease, especially those with oligometastatic tumors [12-15]. However, the overall benefit of surgery in this context continues to be debated, and the best surgical approach (local destruction, ablation therapy, sublobectomy, or lobectomy) requires further clarification [16].
Due to the rapid progression of lung cancer, standardized mortality rates closely mirror incidence rates for both sexes [17]. Unfortunately, over 75% of cases are diagnosed at advanced stages (IIIA-IV) [18]. While radiotherapy and chemotherapy, alone or in combination, offer significant improvements in long-term survival and symptom control for patients with locally advanced or metastatic disease [19, 20], surgery continues to play a crucial role in the management of advanced NSCLC.
Mounting evidence suggests potential survival benefits for carefully selected patients with advanced-stage lung cancer who undergo surgical resection. Studies have demonstrated improved survival outcomes in specific cases of stage IV NSCLC following surgery [21-23]. Notably, a large-scale analysis by Yang et al. in the United States revealed a significant 25% improvement in 5-year overall survival for patients with cT1-2, N0-1, M1 or cT3, N0, M1 disease who underwent surgery compared to those receiving non-surgical treatments (chemoradiation: 5.8%, chemotherapy: 5.9%, radiotherapy: 3.2%) [24].
Bauman JE et al. retrospectively investigated salvage lung resection in 24 stage IIIB patients. Procedures demonstrated technical feasibility with acceptable toxicity, even when performed after a delay following definitive radiotherapy. Key findings included a mean surgical duration of 5.5 hours, average blood loss of 250 ml, and a hospital stay of 8 days. While in-hospital mortality was 4% (morbidity 58%), the median overall survival of 30 months, and an estimated 3-year survival rate of 47% highlight the potential of this approach. Though encouraging, Bauman et al. emphasize the need for prospective validation in a well-defined patient population to determine the true efficacy of this strategy [25].
Sonnet J et al. prospectively evaluated the feasibility and safety of lung resection following induction therapy with concurrent chemoradiation at 45 Gy. The study included 40 patients with diverse preoperative stages (IIb-IV) and a significant proportion (13) with Pancoast tumors. Notably, the procedure was associated with no postoperative deaths. Importantly, pathological analysis revealed a high rate of complete response (45%) and significant reductions in residual disease burden (82.5% with no lymph node involvement). While overall and disease-free survival rates were promising (1 and 5-year rates exceeding 46%), the authors acknowledge the need for confirmation through larger, multi-institutional trials [26].
While surgical intervention offers clear benefits in early-stage NSCLC, its feasibility in stage III-IV disease remains a subject of debate [27]. Prognosis in resectable stage III NSCLC patients undergoing surgery following neoadjuvant therapy is strongly linked to lymph node invasion [28]. Furthermore, stage IV NSCLC typically carries a limited life expectancy, leading to general discouragement for surgical intervention [29, 30].
In the United States, despite a significant improvement in the 5-year survival rate of NSCLC patients from 16.4% to 25.1% (1975-2015), nearly 55% eventually progress to advanced stages [11, 30]. While surgery offers proven benefits in early-stage NSCLC (stages I-II), its feasibility, and effectiveness in stages III-IV remain controversial [27]. For resectable stage III NSCLC, post-neoadjuvant therapy prognosis heavily depends on lymph node invasion [28]. Moreover, surgical intervention is often considered unsuitable in stage IV NSCLC due to limited life expectancy [29].
Ren J et al. demonstrated potential palliative benefits of surgery in stage IV NSCLC, revealing a doubling of average survival compared to the non-surgical group. Cox regression analysis identified surgery as an independent predictor of improved overall survival (OS) (HR=0.441; 95%CI: 0.426-0.456; p<0.001) and cancer-specific survival (CSS) (HR=0.397; 95%CI: 0.380-0.414; p<0.001). Importantly, the study suggests that lobectomy may offer survival advantages over local destruction or sub-lobectomy in this patient population (p< 0.001) [31].
Liang et al. observed a survival benefit in metastatic NSCLC patients who underwent prior primary tumor resection. Their model explored the hypothesis that surgical benefits in stage IV disease may depend on specific patient and tumor characteristics. Analysis of 30.342 stage IV patients revealed that 8.03% underwent primary resection, which was independently associated with a longer cancer-specific median survival (CSS) compared to non-surgical management (19 vs. 9 months, p<0.001). Importantly, 56.40% of the surgical cohort survived beyond 9 months [13].
Prehabilitation, a recently established practice, aims to optimize a patient's functional capacity before surgery and improve post-operative outcomes. This multifactorial approach encompasses medical optimization, exercise training, nutritional counseling, and psychological support to address perioperative stressors [32]. Surgical trauma, anesthesia, and perioperative therapies (neoadjuvant treatment, perfusion, ventilation) all contribute to stress alongside factors like malnutrition and anxiety. Patient resilience to these stressors depends on modifiable factors (comorbidities, smoking, physical fitness, psychological state) and non-modifiable factors (age, gender, cancer biology). Modifiable factors further interact with those related to the underlying malignancy, such as cachexia, malabsorption, and muscle wasting [32].
The efficacy of prehabilitation programs and their optimal duration remain areas of ongoing investigation. However, prehabilitation has the potential to expedite postoperative recovery, enhance quality of life, and improve tolerance to neoadjuvant therapies like chemotherapy [33]. Notably, prehabilitation is not a novel concept in thoracic surgery, where pulmonary prehabilitation has been implemented to improve functional capacity and reduce complications in high-risk patients undergoing lung transplantation or lung volume reduction surgery [34, 35].
In a pioneering study, Sekine et al. (2005) prospectively investigated the impact of a pulmonary prehabilitation program on 22 Chronic Obstructive Pulmonary Disease (COPD) patients undergoing lobectomy (FEV1/FVC ≤70%, >50%) [36]. When compared to a historical control group (n=60) matched for selection criteria, the prehabilitation group demonstrated a significantly lower incidence of postoperative pulmonary complications and a shorter hospital stay.
Jones et al. investigated 25 lung cancer patients, demonstrating significant improvements in VO2 max (3.3 ml/kg/min, p=0.006) and 6-minute walk distance (49 m, p=0.013) after training up to the day of resection [37]. These findings are mirrored in Bobbio et al.'s prospective study of 12 stage I/II NSCLC patients with COPD and compromised VO2 max (≤15 ml/kg/min). A 4-week pulmonary prehabilitation program yielded an average VO2 max increase of 2.8 ml/kg/min [38].
Tarumi et al. demonstrated the potential benefits of pulmonary prehabilitation initiated during induction chemoradiotherapy for lung cancer. Their study of 82 patients revealed significant improvements in both FVC (+6.4%, p=0.0096) and FEV1 (+10.4%, p<0.001) following participation in the 10-week program. Importantly, the most pronounced gains were observed in patients with initial respiratory compromise (FVC <80% or FEV1/FVC <70%), who showed a substantial increase in FVC (+13.9%, p=0.0025) and FEV1 (+22.5%, p<0.0001) [39].
Benzo et al. investigated the feasibility and impact of pulmonary prehabilitation on postoperative morbidity in patients with moderate-severe COPD undergoing curative lung cancer surgery [40]. Two randomized controlled trials were conducted, comparing prehabilitation programs to usual care. The initial 4-week program proved difficult to implement due to low recruitment. A revised 1-week, twice-daily prehabilitation program enrolled 19 patients. While statistically significant reductions in chest drain duration (mean 4.7 vs. 9.0 days, p=0.03) and prolonged drainage (>7 days) were observed in the prehabilitation group (11% vs. 63%, p=0.03), the study was ultimately limited by a small sample size and short program duration, preventing definitive conclusions about the impact of prehabilitation on postoperative morbidity [40].
Gao et al. (2015) investigated the effects of a preoperative pulmonary prehabilitation program on high-risk patients with resectable lung cancer. In their non-randomized study, 71 patients participated in the program (which included abdominal breathing exercises, respiratory device training, and lower limb resistance training) followed by lobectomy. These patients were compared to a control group of 71 patients who underwent lobectomy with conventional management alone [41].
Boujibar et al. investigated the potential of prehabilitation to improve surgical outcomes and reduce morbidity (as measured by the Clavien-Dindo classification) in patients with resectable lung cancer and VO2 max ≤20 ml/min/kg. Their study compared 19 patients who underwent prehabilitation (exercise, muscle strengthening, education, and smoking cessation) to 19 patients who received standard care. Prehabilitation was associated with a significantly lower postoperative complication rate (42% vs. 80%, p=0.0382), particularly in terms of less severe complications (Clavien-Dindo score ≤2, p=0.0252). However, no difference in hospital stay was observed between groups [42].
Licker et al. conducted a randomized controlled trial investigating the impact of preoperative prehabilitation on patients with operable lung cancer. A total of 151 patients were randomized to either prehabilitation (high-intensity interval training, 2-3 sessions/week for an average of 25 days) or usual care. Primary outcomes were post-operative morbidity and mortality, while secondary outcomes focused on changes in cardiopulmonary exercise and 6-minute walk test performance. Despite significant prehabilitation-driven improvements in VO2 max and 6-minute walk test (6MWT) distance (+15%, p=0.003 and +15%, p<0.001 respectively), no significant difference in overall postoperative complication rates was detected (prehabilitation 35.5% vs. usual care 50.6%, p=0.080). However, a sub-analysis revealed a lower incidence of pulmonary complications in the prehabilitation group (23% vs. 44%, p=0.018) [43].
Licker et al. demonstrated the safety and feasibility of a short-term preoperative training program to improve aerobic performance. However, these improvements did not translate to a significant reduction in overall morbidity-mortality compared to usual care. This finding may be partially attributed to the study's inclusion of all resectable lung cancer patients without stratification by risk, potentially obscuring differences in postoperative complications. Additionally, the high proportion of open thoracotomies (>80%), despite many patients having early-stage disease, is a notable deviation from current practices where Video-Assisted Thoracic Surgery (VATS) is preferred. This factor may complicate the interpretation and generalizability of results [43].
Multimodal prehabilitation aims to optimize patients' resilience against surgical, anesthetic, and perioperative stressors, potentially improving long-term outcomes. While studies have demonstrated feasibility, safety, and improved muscle function, evidence of definitive clinical efficacy remains limited. These initial encouraging results justify the need for large-scale clinical effectiveness studies to fully establish the role and benefits of prehabilitation.
Conclusions
Our findings underscore several urgent priorities in healthcare policy and organization. Early detection through targeted lung cancer screening is crucial, given the late-stage presentation of most cases (94%). Alongside this, population-wide smoking cessation programs, environmental protection measures, and promotion of healthy lifestyles are vital for prevention. Standard chemotherapy and radiotherapy offer limited benefits in advanced lung cancer (stages III and IV), highlighting a pressing need for basic research breakthroughs and novel treatment paradigms.
The short average survival times from diagnosis (1.3-1.4 years) put pressure on basic research in particular. In addition to radio- and chemotherapy, the development of anti-tumour immunotherapy is seen as a new line in the non-surgical treatment of lung cancer.
Competing interests
None declared.
Authors’ contributions
Concept and design of study – IM; acquisition of data – IM, SG, IB; analysis and/or interpretation of data – IM, SG, IB; drafting the manuscript – IM, SG; revising the manuscript critically for important intellectual content – IM, SG. All authors have read and approved the final version of the manuscript.
Acknowledgements and funding
The study had no external funding.
Patient consent
Obtained.
Ethics approval
The study protocol was approved by the Research Ethics Committee of Nicolae Testemiţanu State University of Medicine and Pharmacy (Protocol No. 04 of 12.11.2020).
Authors’ ORCID IDs
Igor Maxim – https://orcid.org/0009-0002-9356-4368
Serghei Guțu – https://orcid.org/0000-0001-9583-0485
Ion Burlacu – https://orcid.org/0009-0002-4160-9173
References
Li C, Lei S, Ding L, et al. Global burden and trends of lung cancer incidence and mortality. Chin Med J (Engl). 2023;136(13):1583-1590. doi: 10.1097/CM9.0000000000002529.
Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021. doi: 10.1002/ijc.33588.
World Health Organization (WHO). Global health estimates 2020: deaths by cause, age, sex, by country and by region, 2000-2019 [Internet]. Geneva: WHO; 2020 [cited 2020 Dec 11]. Available from: who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death.
Shack L, Jordan C, Thomson CS, Mak V, Møller H; UK Association of Cancer Registries. Variation in incidence of breast, lung and cervical cancer and malignant melanoma of skin by socioeconomic group in England. BMC Cancer. 2008;8:271. doi: 10.1186/1471-2407-8-271.
Lackey A, Donington JS. Surgical management of lung cancer. Semin Intervent Radiol. 2013;30(2):133-140. doi: 10.1055/s-0033-1342954.
Petrella F, Rizzo S, Casiraghi M, et al. State of the art and new perspectives in surgical treatment of lung cancer: a narrative review. Transl Cancer Res. 2022;11(10):3869-3875. doi:10.21037/tcr-22-1491.
Couñago F, Navarro-Martin A, Luna J, et al. GOECP/SEOR clinical recommendations for lung cancer radiotherapy during the COVID-19 pandemic. World J Clin Oncol. 2020;11(8):510-527. doi: 10.5306/wjco.v11.i8.510.
Shakya P, Poudel S. Prehabilitation in patients before major surgery: a review article. JNMA J Nepal Med Assoc. 2022;60(254):909-915. doi: 10.31729/jnma.7545.
Molenaar CJL, Papen-Botterhuis NE, Herrle F, Slooter GD. Prehabilitation, making patients fit for surgery - a new frontier in perioperative care. Innov Surg Sci. 2019;4(4):132-138. doi: 10.1515/iss-2019-0017.
Rjabov AB, Pikin OV, Glushko VA, Kolbanov KI, Bagrov VA, Aleksandrov OA, Barmin VV, Martynova DE, Vorobyeva EY, Larionov DA. Khirurgicheskoe lechenie raka legkogo u bol'nykh starshe 75 let [Surgical treatment of lung cancer in patients over 75 years old]. Khirurgiia (Mosk). 2022;(12):20-30. Russian. doi: 10.17116/hirurgia202212120.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33. doi: 10.3322/caac.21654. Erratum in CA Cancer J Clin. 2021 Jul;71(4):359.
Opitz I. Commentary: Surgery expanding to stage IV non-small cell lung cancer treatment?! J Thorac Cardiovasc Surg. 2021;161(4):1508-1509. doi: 10.1016/j.jtcvs.2020.03.054.
Liang H, Liu Z, Huang J, et al. Identifying optimal candidates for primary tumor resection among metastatic non-small cell lung cancer patients: a population-based predictive model. Transl Lung Cancer Res. 2021;10(1):279-291. doi: 10.21037/tlcr-20-709.
Coster JN, Groth SS. Surgery for locally advanced and oligometastatic non-small cell lung cancer. Surg Oncol Clin N Am. 2020;29(4):543-554. doi: 10.1016/j.soc.2020.07.001.
David EA, Andersen SW, Beckett LA, et al. Survival benefits associated with surgery for advanced non-small cell lung cancer. J Thorac Cardiovasc Surg. 2019;157(4):1620-1628. doi: 10.1016/j.jtcvs.2018.10.140.
Collaud S, Stahel R, Inci I, et al. Survival of patients treated surgically for synchronous single-organ metastatic NSCLC and advanced pathologic TN stage. Lung Cancer. 2012;78(3):234-238. doi: 10.1016/j.lungcan.2012.09.011.
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374-1403. doi: 10.1016/j.ejca.2012.12.027
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43-66. doi: 10.3322/canjclin.57.1.43
Laine AM, Westover KD, Choy H. Radiation therapy as a backbone of treatment of locally advanced non-small cell lung cancer. Semin Oncol. 2014;41(1):57-68. doi: 10.1053/j.seminoncol.2013.12.012.
Non-small Cell Lung Cancer Collaborative Group. Chemotherapy for non-small cell lung cancer. Cochrane Database Syst Rev. 2000;2000(2):CD002139. doi: 10.1002/14651858.CD002139.
Hanagiri T, Takenaka M, Oka S, et al. Results of a surgical resection for patients with stage IV non--small-cell lung cancer. Clin Lung Cancer. 2012;13(3):220-224. doi: 10.1016/j.cllc.2011.05.006
Kawano D, Takeo S, Katsura M, Tsukamoto S, Masuyama E, Nakaji Y. Surgical treatment of stage IV non-small cell lung cancer. Interact Cardiovasc Thorac Surg. 2012;14(2):167-170. doi: 10.1093/icvts/ivr036.
David EA, Canter RJ, Chen Y, Cooke DT, Cress RD. Surgical management of advanced non-small cell lung cancer is decreasing but is associated with improved survival. Ann Thorac Surg. 2016;102(4):1101-1109. doi: 10.1016/j.athoracsur.2016.04.058.
Yang CJ, Gu L, Shah SA, et al. Long-term outcomes of surgical resection for stage IV non-small-cell lung cancer: a national analysis. Lung Cancer. 2018;115:75-83. doi: 10.1016/j.lungcan.2017.11.021.
Bauman JE, Mulligan MS, Martins RG, Kurland BF, Eaton KD, Wood DE. Salvage lung resection after definitive radiation (>59 Gy) for non-small cell lung cancer: surgical and oncologic outcomes. Ann Thorac Surg. 2008;86(5):1632-1639. doi: 10.1016/j.athoracsur.2008.07.042.
Sonett JR, Suntharalingam M, Edelman MJ, et al. Pulmonary resection after curative intent radiotherapy (>59 Gy) and concurrent chemotherapy in non-small-cell lung cancer. Ann Thorac Surg. 2004;78(4):1200-1206. doi: 10.1016/j.athoracsur.2004.04.085.
Yokoi K, Taniguchi T, Usami N, Kawaguchi K, Fukui T, Ishiguro F. Surgical management of locally advanced lung cancer. Gen Thorac Cardiovasc Surg. 2014;62(9):522-530. doi: 10.1007/s11748-014-0425-7.
Donington JS, Pass HI. Surgical approach to locally advanced non-small cell lung cancer. Cancer J. 2013;19(3):217-221. doi: 10.1097/PPO.0b013e318299f647.
Roy MS, Donington JS. Management of locally advanced non small cell lung cancer from a surgical perspective. Curr Treat Options Oncol. 2007;8(1):1-14. doi: 10.1007/s11864-007-0023-3.
Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. (eds). SEER Cancer Statistics Review, 1975-2016 [Internet]. Bethesda: National Cancer Institute; 2018 [cited 2023 Oct 19]. Available from: https://seer.cancer.gov/archive/csr/1975_2016/index.html
Ren J, Ren J, Wang K, Tan Q. The consideration of surgery on primary lesion of advanced non-small cell lung cancer. BMC Pulm Med. 2023;23(1):118. doi: 10.1186/s12890-023-02411-w.
Levett DZ, Edwards M, Grocott M, Mythen M. Preparing the patient for surgery to improve outcomes. Best Pract Res Clin Anaesthesiol. 2016;30(2):145-157. doi: 10.1016/j.bpa.2016.04.002.
Ryan AM, Power DG, Daly L, Cushen SJ, Ní Bhuachalla Ē, Prado CM. Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc. 2016;75(2):199-211. doi: 10.1017/S002966511500419X.
Wickerson L, Rozenberg D, Janaudis-Ferreira T, et al. Physical rehabilitation for lung transplant candidates and recipients: an evidence-informed clinical approach. World J Transplant. 2016;6(3):517-531. doi: 10.5500/wjt.v6.i3.517.
Rochester CL. Pulmonary rehabilitation for patients who undergo lung-volume-reduction surgery or lung transplantation. Respir Care. 2008;53(9):1196-1202.
Sekine Y, Chiyo M, Iwata T, et al. Perioperative rehabilitation and physiotherapy for lung cancer patients with chronic obstructive pulmonary disease. Jpn J Thorac Cardiovasc Surg. 2005;53(5):237-243. doi: 10.1007/s11748-005-0032-8.
Jones LW, Peddle CJ, Eves ND, et al. Effects of presurgical exercise training on cardiorespiratory fitness among patients undergoing thoracic surgery for malignant lung lesions. Cancer. 2007;110(3):590-598. doi: 10.1002/cncr.22830.
Bobbio A, Chetta A, Ampollini L, et al. Preoperative pulmonary rehabilitation in patients undergoing lung resection for non-small cell lung cancer. Eur J Cardiothorac Surg. 2008;33(1):95-98. doi: 10.1016/j.ejcts.2007.10.003.
Tarumi S, Yokomise H, Gotoh M, et al. Pulmonary rehabilitation during induction chemoradiotherapy for lung cancer improves pulmonary function. J Thorac Cardiovasc Surg. 2015;149(2):569-573. doi: 10.1016/j.jtcvs.2014.09.123.
Benzo R, Wigle D, Novotny P, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer. 2011;74(3):441-445. doi: 10.1016/j.lungcan.2011.05.011.
Gao K, Yu PM, Su JH, et al. Cardiopulmonary exercise testing screening and pre-operative pulmonary rehabilitation reduce postoperative complications and improve fast-track recovery after lung cancer surgery: a study for 342 cases. Thorac Cancer. 2015;6(4):443-449. doi: 10.1111/1759-7714.12199.
Boujibar F, Bonnevie T, Debeaumont D, et al. Impact of prehabilitation on morbidity and mortality after pulmonary lobectomy by minimally invasive surgery: a cohort study. J Thorac Dis. 2018;10(4):2240-2248. doi: 10.21037/jtd.2018.03.161.
Licker M, Karenovics W, Diaper J, et al. Short-term preoperative high-intensity interval training in patients awaiting lung cancer surgery: a randomized controlled trial. J Thorac Oncol. 2017;12(2):323-333. doi: 10.1016/j.jtho.2016.09.125.