Introduction
Every human being, as a citizen of a certain state, lives and works in a certain legal area and in a certain ethical-moral comfort zone; therefore, the importance and role of legal and ethical regulations increase significantly. The state of his health and quality of life will also depend, in these cases, on how well coordinated, harmonious, effective, and promptly enforced moral, ethical, professional, and legal rules will work to defend the rights of citizens with this special status.
Health, medicine, and pharmacy are three interdependent components that can influence the quality of life of any individual. Medicine and pharmacy are also integrated parts of health care, their basic task being to ensure, maintain, and preserve human (and animal) health. Both simultaneously manifest, preserve, and guarantee freedom of expression, both in terms of professional ethics and deontology and in terms of the legal sciences. The quality of the medical and pharmaceutical acts provides a constructive and independent dialogue between all health professionals and the consumers of medicines, the patients, with a direct benefit for the latter. In this context, Professor Safta V. defines the ultimate goal of the pharmaceutical act as „ensuring the achievement of the ultimate goal of the health system through the active involvement of the pharmacist in providing effective, harmless, and accessible medicines and medical-pharmaceutical products for the treatment process of each individual and the entire society” [1]. Analyzing the sources of legal regulation of social relations concerning the protection of health and the protection of the rights of citizens with this special status, we note the positions of many authors who also emphasize the role of moral norms, ethics, and professional deontology, both in medicine and pharmacy [2-4]. And we propose that every time, when investigating abuses and violations in the field of health protection, the rights of the consumer of medicines, the patient, should also be approached through the prism of assessing the place and role of ethical norms, which generally have the same historical-legal origin, thematic orientation, and/or direct influence on the law and the practice of law enforcement.
The sources of law in the relations considered are a well-defined system of legal normative acts, which include, first and foremost, legal rules governing social relations regarding the protection of public health in general but also the protection of the health of individual citizens.
Material and methods
A secondary descriptive study of normative legal acts on the functionality of the „moral-ethics-deontology-law” system was carried out through the prism of the protection of the rights of the patient, the final beneficiary of social relations in the field of health care, as primary specific non-traditional sources.
The study was carried out from November 2016 to December 2021 and covered the period from 1991 to 2021. It involved data selection and systematization, a systematic approach to the literature and internet sources, and their analysis via Google search engine queries, Google Scholar using keywords appropriate to the topic, and manual evaluation of returned articles. The purpose was to identify the essence and functionality of the system „moral-ethics-deontology-law” in the protection of the rights of the consumer of medicines, the patient - the final beneficiary of social relations in the field of health care - and the description of the non-traditional sources of pharmaceutical law. The most relevant sources to analyze were the Constitution of the Republic of Moldova, the Code of Ethics for doctors and pharmacists in the Republic of Moldova, laws and sub-legislative acts, and various relevant bibliographic sources. The study is based on the use of several recognized techniques and methods of analysis: systemic approach, analysis and synthesis, logical-legal deduction, comparative analysis, etc.
Results
What constitutes law in the health-care system is still an open question, and legal doctrine has yet to provide an answer. The conclusion that the protection of citizens' health is achieved by means of legal rules in various branches of law is nothing other than an acknowledgement of the current situation, which is binding on nothing and nobody. Many health-care laws are quite complex legal documents that include rules from civil, criminal, administrative, tax, and other areas of law.
Moral, ethical, and bioethical rules: the sources of medico-pharmaceutical law
If social relations in the field of health care are regulated by various branches of law and not by a single branch, there is a real risk of confusing the concepts of „law” and „legislation” applicable to this field. To reduce this risk, it is first necessary to determine the mutual interactions between morality, ethics, and law and, second, to determine the hierarchy by recognizing their importance in medical, pharmaceutical, and legal practice.
„Morality” is defined as „the attribute of what is moral; the nature, character, and value of an event or the conduct of a person or a community from a moral standpoint; behavior, conduct, and morals in accordance with moral principles; honesty, good conduct - from lat. moralitas, -atis, fr. moralité” by dexonline.ro. Similarly, morality also determines the spiritual qualities necessary for a person in society [5].
Ethics, according to the same dictionary, is „the theoretical study of human values and conduct in the light of moral principles and their role in social life; the totality of the corresponding rules of moral conduct; morality - from fr. éthique, lat. ethicus”. Similarly, ethics is the science of morality, its meaning, principles, norms, and role in society, as well as the totality of norms of behavior, the morality of social groups, and professions.
Thus, medical and pharmaceutical ethics investigates and defines the value of the doctor and pharmacist's professional activities to society, as well as their personal characteristics. In this context, Professor Baciu Gh. mentions the position of the physician Albert Schweitzer (1875–1965), winner of the Nobel Peace Prize in 1952, who defined ethics as „respect for any life”. By means of this universal respect, man encounters the world and is in harmony with its laws. Such a principle can lead to a profound and universal humanism, which must be the dominant element in the contemporary world [2].
In his book „Bioethics: Origins, Dilemmas, Trends,” Professor Țîrdea T. mentions that „the term ethics comes from the Greek word „ethos,” which means „character,” „conduct,” and „custom.” The Romans derived the adjective moralis (moralitas) from the word mos (mores), which means „habit” or „custom” in Latin. Therefore, these two words, „ethics” and „morality,” etymologically coincide. Although originally the two nominalized terms, one Greek and the other Latin, had approximately the same meaning, their etymological evolution dissociated them, giving them different meanings [3].
In other words, if ethics is „character,” „conduct,” or „custom,” then morality, according to the generally accepted conception, is how people understand the notion of right and/or wrong, „right” and „wrong” behavior. The norms and rules are formed based on these understandings, without which one cannot live in a society, community, etc.
The same dictionary, dexonline.ro [5], defines bioethics as the morality of science in general and medicine in particular, which prohibits the commercialization of the human body and organ trafficking (from fr. bioéthique, it. bioetica). Similarly, bioethics is a scientific discipline and a sphere of practical activity concerning the non-formal regulation of health care relationships. Bioethics is a much broader concept, its subject being the human attitude towards all living things. Bioethics „derives” from medical ethics, and through this „prism,” it can be understood as part of professional medical and pharmaceutical ethics.
According to some scholars, which we also support, modern bioethics is defined by three representative levels: the theoretical level, the practical level, and the applied level. Theoretical bioethics represents the totality of knowledge about human attitudes towards all living things, expressed in the form of an axiological discourse. The institutionally formed regulation and value expertise of human attitudes toward all living things is known as „practical bioethics.” These stipulations are duly formulated in the form of oaths, documents, and declarations, which, in essence, have no legal character. Applied bioethics is a description of specific cases and specific situations of human behavior in relation to all living things [3, 6].
Not only to promote a new science subfield in the Republic of Moldova - namely pharmaceutical law and medical law - but rather for the purpose of the present research, it is important to note that bioethics is a part of ethics (morality), being determined by some objective criteria such as profession, professional activity, and vocation. Bioethics, as a component part of ethics in the studied context, is intended to study and highlight the peculiarities of the moral component not only of the systems „patient-doctor”, „patient-pharmacist”, „doctor-doctor”, „pharmacist-pharmacist”, but also of the specific systems „drug consumer-pharmacist”, „drug consumer-doctor-pharmacist” [7, 8]. The essential specific features observed in some cases recently require us to propose a new configuration of this system, namely: „consumer of medicines - doctor -pharmacist - lawyer.”
Both medicine and pharmacy, from ancient times, have been most closely linked to the rules of morality. They began as public regulatory instruments that were very close to legal rules. At the same time, if law is a system of order in society endowed by the state with a constraining (coercive) force, then morality is a system of visions and reflections that possesses no such force. The systemic approach to contrasting law and morality enables a much deeper understanding of the content of these phenomena.
The organic link between morality and law–spheres of human life
Analyzing law and morality according to their scope, it can be seen that these notions are not similar: morality covers all sides of human behavior, while law covers only the most important social relations, those that require constraint measures by the state [9]. Consequently, the sphere of moral (ethical, bioethical) influence on an individual's behavior shows itself to be much wider than the sphere of legal regulation.
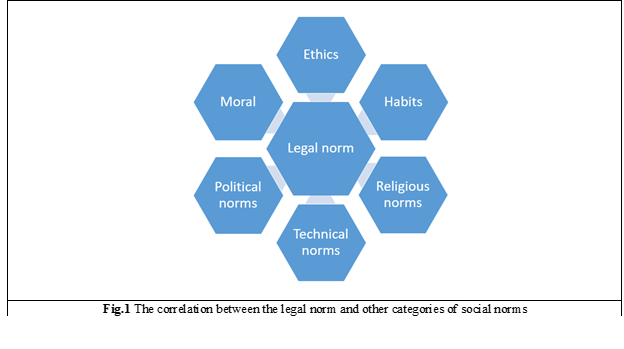
According to the influenced object, the concepts of law and morality in general are similar. Both law and morality involve the whole of society, all groups, and all categories of the population. On the other hand, as we know, different groups may simultaneously share different views of morality. The correlation of the legal norm with other categories of social norms is shown in Figure 1.
Legal and moral norms do not always, but frequently, coincide in content. Law is part of morality, but with the presence of a specific instrument - the force of coercion. At the same time, hypothetically, the adoption of unconstitutional rules cannot be ruled out. The practices of the Constitutional Court and the Parliament of the Republic of Moldova clearly prove this. Law and legal norms are only moral when they result from a social compromise; therefore, there may be contradictions between moral norms (ethics), which over time evolve either in favor of morality or in favor of law (legal norm), forming new values, provisions, regulations, and beliefs [10, 11].
Depending on the form of manifestation, moral and legal norms can be very different. Legal norms are promoted by means of decisions, acts of state power, administrative bodies, and courts. The special procedure of state registration is a feature of legal norms, while moral norms have no such characteristic. In terms of bioethical norms, it should be noted that some norms and regulations, particularly their common parts, do not require special registration, whereas others do. Various legislative acts can serve as examples, such as:
The Code of Ethics for doctors and pharmacists, adopted by Government Decision No. 192 of 24.03.2017, based on the current legislation of the Republic of Moldova;
Law no.261 of 01.11. 2013 about the College of Physicians of the Republic of Moldova, which in Article 4 provides that „in the field of training and professional development of physicians, the College of Physicians: 1) participates, through endorsement, in the development and approval of policies in the field of training and professional development of physicians, namely: a) submits to the Ministry of Health of the Republic of Moldova proposals on the amendment and completion of the Framework Code of Ethics (deontology) of the medical and pharmaceutical worker; 4) a) examines petitions and addresses of natural and legal persons exclusively on cases of deviations of physicians from the rules of professional ethics, medical deontology and the rules of good professional practice...”, but does not specify who, which of the specialist associations participates in the drafting of the rules of medical ethics and the settlement of cases of their violation. We assume that it is a question of setting up specialized collegial bodies - the College of Physicians, the College of Pharmacists, the College of Dentists, etc. - that would, in our opinion, be the most competent bodies in drafting, promoting, and assessing compliance with the rules concerned.
Looking at how bioethical norms influence legal regulation in the health sphere, including pharmaceutical activity in general, not only in the Republic of Moldova but also in other countries, it can be seen that they go through a series of stages, the first being always the stage of regulating relations in the public sector. It includes both the drafting of legal rules and their adoption. Moral and ethical rules can be the subject of legal science studies in order to determine the interests of various communities and professional groups and to formulate legislative ideas and legal formulations on their basis. Bioethical rules, therefore, directly or indirectly, through a different degree of involvement, can influence the content of the actual law at any stage of the historical development of social relations in the system of „drug consumer-doctor” or „drug consumer-pharmacist.” As an example, consider the National Health and Medicines Policies and Strategies In the Republic of Moldova, as in other countries, the National Health Policy 2007–2021 represented a set of priorities and development directions in the field of health, established for 15 years with the aim of strengthening the health of the population and reducing inequalities between different social groups and regions of the country [12]. The aim of the National Health Policy was „to create optimal conditions for the maximum realization of the health potential of each individual throughout life and to achieve adequate standards of quality of life for the population.” The general objectives of the National Health Policy are: (1) to increase life expectancy at birth and increase the duration of healthy life; (2) to ensure quality of life and reduce differences in terms of health for all social groups; (3) to strengthen intersectoral partnerships aimed at strengthening the health of the population; and (4) to make individuals responsible for their own health [13].
The State Policy of the Republic of Moldova in the field of medicine stated „the development of the health system in the Republic of Moldova requires health care for citizens on equal principles” [14]. Medicines are an important element in the prophylaxis, diagnosis, and treatment of diseases. The coordinated development of the pharmaceutical sector, especially in relation to its social importance, is one of the priority issues in health care. State policy in the field of medicines is an important component of the National Health Policy. The policy will serve as the foundation for the elaboration of programs for the development of the Republic of Moldova's pharmaceutical system (development, testing, authorization, manufacture, distribution, and rational use of medicines), as well as legislation governing medicines and pharmaceutical activity. We regret that this policy document has been ignored; furthermore, we believe that this guiding document for the development of legislation in the field of medicines and pharmaceutical activity has fallen into disuse.
In Romania, the principle on the basis of which the purpose of the National Health Strategies (2001) [15], as well as the purpose of the National Medicines Policy, was established, is that laid down in the Romanian Constitution [16]. According to this principle, the state is obliged to guarantee, through specific laws and regulations, the population's right to health services.
In other countries, the legislative bases on health protection state that: „the protection of citizens' health includes all political, economic, social, legal, cultural, scientific, medical, sanitary-hygienic and anti-epidemic measures aimed at protecting and strengthening the physical and mental health of each person, supporting the long life of active people, providing them with the necessary medical care in case of loss of health” [6].
Although there is direct interaction between medicine and law, medicine and bioethics in all of these acts, medicine remains both a component of its own system (health care) and a component of the larger system (law-medicine-bioethics).
One of the most eloquent examples of the legal assimilation of ethical norms is Article 9 of the Law concerning the Practice of the Medical Profession, No. 264 of 27.10.2005, according to which graduates of medical and pharmaceutical institutions of high education shall take the doctor's oath [7]. A simple analysis of the text reveals an analogy with the Hippocratic oath, known for hundreds of years to doctors throughout the world. In addition, in other countries, under the legislation in force, doctors bear legal responsibility for violating this oath [6].
It should also be noted that some bioethical documents are legally binding worldwide. For example, if a state accedes to the Convention on Biomedicine, the rules of the „Convention” become binding on that state [17].
Moral and legal principles - guarantees for the realization of the sources of medico-pharmaceutical law
Regarding the protection of the rights of the consumer of medicinal products, the principles of bioethics include the millenary experience of generations and express their unconditional recognition, used equally by both legal doctrine and the lawmaker, who as such can become specific non-traditional sources. Thus, on the one hand, the principles of bioethics represent, in relation to the content of the rules, legal customs, and on the other, the legal provisions outline the framework within which the principles of bioethics can operate.
The principle of patient autonomy (except in cases of self-medication) can be used as an example of compliance by the consumer of medicinal products with one of the basic principles of bioethics:
In its most general form, it entails the consumer of medicinal products being asked for consent to treatment from the stage of prescribing the medicinal product, based on good information practice (and enshrined in law), with the doctor having the obligation to propose to the consumer of medicinal products all options for medicinal treatment, with justification and a forecast of the consequences. Thus, based on this bioethical principle, the doctor cannot prescribe a medicine in any case; instead, he should first propose to the drug user all possible options for drug treatment, including details of efficacy (bioavailability), contraindications, adverse effects, mode of administration, and so on, which will enable the drug consumer, together with the doctor, to identify, on the basis of their individual characteristics (age, sex, psychophysiological condition, social, professional, national, economic, religious status, etc.), their „medicine” (personalized medication);
The principle of the autonomy of the will (consent) of the consumer of medicinal products simultaneously manifests itself as a legal principle. It has obtained legislative recognition in the Order of the Ministry of Health of the Republic of Moldova no. 303 of May 6, 2010 on ensuring access to information about medical data and the list of medical interventions requiring informed consent: p. II, „Parental interventions, including immunizations”; p. VI, „Therapeutic treatments with specific adverse effects or increased risk” (Annex 2);
The principle of autonomy of will (agreement) of the consumer of medicinal products is a special one in relation to the principle of autonomy of will, characteristic of civil law, legislated in the Civil Code of the Republic of Moldova (CCRM), namely Art. 1, Book I, Title I, Chapter I of the CCRM, which states: „(1) Civil legislation is based on the recognition of the participants' equality in the relations regulated by it, the inviolability of property, freedom of contract, the inadmissibility of interference in private affairs, the need for the free realization of civil rights, the guarantee of the restoration of the person's rights (in case of violation), and the judicial defense. (2) Natural and legal persons shall be free to establish by contract their rights and obligations and other contractual conditions, provided that they do not contradict the law”. Article 21 of the CCRM defines the notion of „consumer”: (1) A consumer is any natural person who, in a civil legal relationship, acts predominantly for purposes that are not entrepreneurial or professional. A natural person is not a consumer if the other party to the civil legal relationship is not a professional. (2) Any natural or legal person governed by public or private law who, in a civil law relationship, is acting for purposes relating to his trade, business, or profession, even if that person is not acting for purposes relating to his trade, business, or profession for profit, shall be regarded as a trader. In general, civil law provides that „citizens (physical persons) and legal persons acquire and exercise their civil rights by their own will and in their own interest” [3].
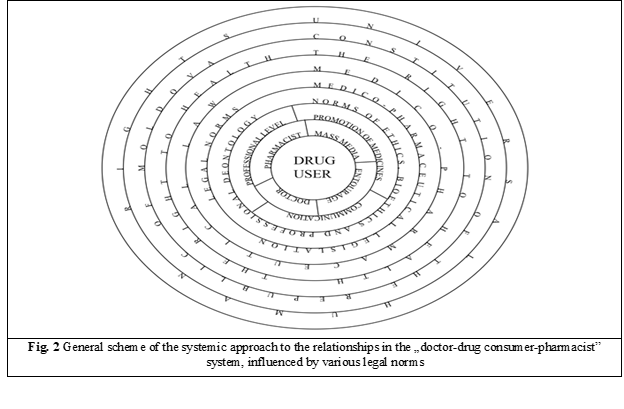
Based on the postulates and principles set out above, the general outline of the systemic approach to the „doctor-drug user-pharmacist” correlations influenced by various legal rules is proposed (Figure 2).
Unquestionably, one of the main sources of ethical regulation of the behavior of the medical and pharmaceutical bodies in the Republic of Moldova is the Code of Ethics for doctors and pharmacists, adopted by Government Decision No. 192 of 24.03.2017 [18]. According to p.46, Section III of the Code, „Consent will be accepted only after fully informing the patient about the diagnosis, prognosis, therapeutic alternatives, their risks, and benefits (the primary objective being the life, health, and benefit of the patient).” Point 53, Section IV of the Code, also sets out patients' rights to medical confidentiality. Medical workers and pharmacists have a duty to protect the confidentiality of information about patients obtained during their professional activity through the processes of accumulating, storing, transmitting, receiving, or destroying personal data.
Stages in the crystallization of medico-pharmaceutical law
As can be seen, ethical rules also have a visible impact on doctrine and case law. A number of such rules are also incorporated into legal norms, acquiring obligatory features and other features inherent in the legal norm. We understand and support the idea that their main function should be „to help those involved in the public health sphere avoid misunderstandings and violations, to point out the best ways to make decisions without inducing prejudice and accusations, to protect both the dignity of the consumer of medicines and the personal dignity of the doctors and pharmacists, who are daily burdened with the responsibility of making decisions and can become the target of severe and often unfounded accusations, and to overcome the conflicts between personalized, new, technological medicine and old ethics” [19].
Considering the results of the study and the opinions of other researchers in the field, we can see four stages of the functionality of the „morals-ethics-deontology-law” system, which can be applied to the field of pharmaceutical law: the theoretical stage, the applied (practical) stage, the scientific stage, and the teaching stage (Figure 3).
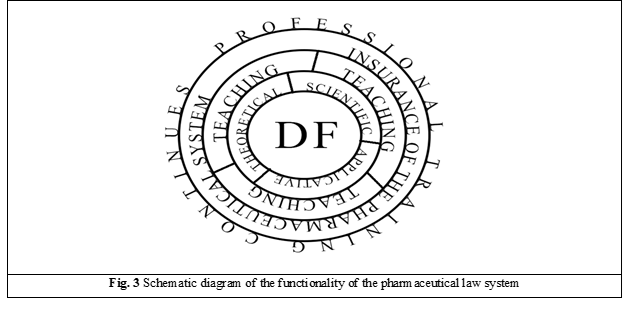
The first stage of the interaction between bioethics and pharmaceutical law is at the theoretical level, involving the use of existing ideas, concepts, and theories in the fields concerned.
The second stage is the application level, which involves:
the emergence of legal relations through the formation of certain (guided) behaviors on the part of the consumer of medicines. At this stage, legal and ethical norms interact synchronously, forming an optimal social variant specific to relations and behavior in the health care system. Inevitably, they must ensure that the fundamental rights and obligations of both the consumer of medicines (the patient) and the healthcare workers are realized. In this particular case, the area of ethical evaluation is much wider than the legal area, since law regulates not all but only some of the relations within this functional system, which allows it to be a kind of law laboratory at the same time. Thus, with the emergence of partnership relations between doctor, pharmacist, and patient and the spread of the rules of the Civil Code on services and medical services, there is also the real possibility, with the agreement of the parties, of fixing the ethical rule in the provisions of the contract. Such a norm, obtaining the „residence visa” among the specific clauses of a contract for the provision of medical and/or pharmaceutical services, will acquire the status of an obligation for the parties and will no longer be accepted only as a purely ethical rule;
the development of legal rules in the knowledge that selected individuals (based on legal and bioethical rules) can achieve a „model of behavior,” often being totally „indifferent” to legal rules, only if there are no deviations from the chosen model;
application of the law, which is not mandatory but characterizes the law as a specific regulator of social relations. The application of coercive measures by the state (of sanctions), expressed in individual prescriptions, is associated with law enforcement.
The third stage is the scientific level, which imposes the need for evidence-based argumentation of the correlations in the „moral-ethics-deontology-law” system applicable to social relations in the „doctor-drug consumer-pharmacist” system, which are strictly oriented towards obtaining health benefits.
The fourth stage is the didactic level, which involves continuous direct and/or distance training of all parties involved in the relationships under study (the „doctor-medicine consumer-pharmacist-lawyer” system).
Conclusions
There is no doubt that fundamental human rights and freedoms are supreme and constitutionally guaranteed values (see articles 1(3) and 16(1) of the Constitution of the Republic of Moldova). The life of the human being as a biological phenomenon acquires the legal status of a fundamental right (Article 24 of the Constitution), with the protection of health as a source of life protection [20].
These qualities are ensured by legal regulations, but as sources of law, they will not be sufficient if they are not supplemented and enriched by the moral, ethical, bioethical, and deontological sources of medical workers and pharmacists. And, while the literature extensively explains the significance of legal sources, much less is exposed and demonstrated about the aptitude of moral, ethical, bioethical, and deontological rules sources.
Through the research we conducted, we were able to identify the dialectical and organic unity between moral, ethical, bioethical, and deontological sources, and legal norms - the moral-legal foundation of medico-pharmaceutical law.
Competenig interests
None declared
Author’s ORCID ID
Alexandru Znagovan - https://orcid.org/0000-0001-9344-8872
References
Safta V. [Analytical highlights on the application of the „end goal” of the systems approach in pharmacy practice]. [Pharm J Mold (Chisinau)]. 2014;(3-4):20. Romanian.
Baciu G. [Ethico-moral and bioethical issues]. Arta Medica (Chisinau). 2011;(1/44):31-34. Romanian.
Țîrdea T. [Bioethics: origins, dilemmas, trends: course support]. Chisinau: Medicina; 2005. 233 p. Romanian.
Safta V, Durbailova A, Zgîrcu E. [Ethical-deontological landmarks of the contemporary pharmaceutical act]. [Pharm J Mold (Chisinau)]. 2015;(1-2):8-17. Romanian.
Dexonline.ro: dictionaries of the Romanian language. Definition of the words: morality, bioethics [Internet]. © 2004-2022 dexonline [cited 2022 Nov 9]. Available from: www.dexonline.ro
Sedova N. [Legal bases of bioethics]. Moscow: Triumph; 2004. 224 p. Russian.
Republic of Moldova, The Parliament. [Law No 264 of 27 October 2005 regarding the practice of the medical profession]. Monitorul Oficial al Republicii Moldova. 2005;(172-175):art. 839. Romanian.
Znagovan A. Legal aspects of medicines consumption - the sources of legal regulation concerning the liability for causing health damages to medicines consumer. In: 21st century pharmacy-between intelligent specialization and social responsibility: Proceedings of the 17th Romanian National Congress of Pharmacy; 2018 Sep 26-29; Bucharest, Romania. Bucharest: Filodiritto; 2018. p. 241-246. ISBN: 978-88-85813-28-1.
Livshitz RZ. Teoriia prava [Theory of law]. Moscow: Bek; 1994. 208 p. Russian.
Cioban T. [Some aspects of parliamentary deontology]. Administrarea Publică (Chisinau). 2010;(2-3):29-36. Romanian.
Constitutional Court of the Republic of Moldova. Declaration of unconstitutionality of a law adopted by holding the Government accountable to the Parliament during the parliamentary recess (Complaint no.28a/2011) [Internet]. Chisinau: The Court; 2011- [cited 2022 Nov 9]. Available from: https://www.constcourt.md/libview.php?l=ro&idc=7&id=128&t=/Media/Noutati/Declararea-neconstitutionala-a-unei-legi-adoptate-prin-angajarea-raspunderii-Guvernului-in-fata-Parlamentului-in-perioada-vacantei-parlamentare. Romanian.
Republic of Moldova, The Government. [Decision No 886 of 6 August 2007 regarding the approval of the National Health Policy]. Monitorul Oficial al Republicii Moldova. 2007 Aug 17;(127-130):art. 931. Romanian.
Znagovan A, Casian N. [Health systems management]. In: [Theory and practice of public administration: Proceedings of the Scientific-Practical Conference of the Academy of Public Administration of the Republic of Moldova; 2012 May 22]. Chisinau; 2012. p. 266-269. ISBN 978-9975-4107-8-6. Romanian.
Republic of Moldova, The Parliament. [Decision No1352 of 03 Oct 2002 on the approval of the State Policy on Medicines]. Monitorul Oficial al Republicii Moldova. 2002;(149):art. 1161. Romanian.
Government of Romania, Ministry of Health and Family. [Relaunching the reform in the field of health: National strategies in the field of health] [Internet]. Snagov; 2001 [cited 2022 Nov 9]. Available from: http://gov.ro/fisiere/programe_fisiere/13-07-30-02-08-443.pdf. Romanian.
Romanian Constitution. Art. 34: The right to health protection [Internet] [cited 2022 Nov 9]. Available from: https://www.constitutia.ro/art-34-dreptul-la-ocrotirea-sanatatii.htm. Romanian
Republic of Moldova, The Government. [Decision No 192 of 24 March 2017 regarding the approval of the Code of ethics of medical workers and pharmacists]. Monitorul Oficial al Republicii Moldova. 2017 Mar 31;(92-102):art. 265. Romanian.
Țîrdea T, Gramma R. [Medical bioethics in public health: Course support]. Chișinău: Bons Offices; 2007. 248 p. Romanian.
Constitution of the Republic of Moldova, 12 July 1994. Monitorul Oficial al Republicii Moldova. 1994 Aug 12;(1). Romanian.