Introduction
As in any human activity, the pharmaceutical activity faces a lot of classic and emerging risks. Numerous organizations and companies in the pharmaceutical field, national and international, are concerned with the issue of risks, risk management being a valuable component of an effective quality system.
Modern risk identification methods have developed from techniques already tested and applied in risk management. For example, the Risk Score Method has its origins in balanced scorecard methods used to quantify the performance of organizations, while the process mapping methodology developed from the field of quality management and is based on techniques from the field of flow charting [1]. Any organization develops on the basis of a variety of processes. By representing these processes in the form of a diagram - their mapping - risk management tries to improve the organization's activity.
This method has its origins in quality management during the process reorganization in the 80's and 90's in the United States of America and Europe. The current's slogan was „if it ain't broke, fix it!”. In this way, even if an organization operates efficiently and effectively, it can benefit from a detailed analysis of processes in order to improve them.
Since then, risk assessment and treatment methods and techniques have developed, covering almost all areas [2].
In 2009, good practice in the field of risk is included in the ISO 31000:2009 standard, which tries to standardize the terminology and concepts in the field of risk, placing a special emphasis on the organizational context, as well as on the description of a risk management process, a process that can be applied only integrated within organizational processes and practices. Subsequently, the risk assessment techniques available at the time are presented in ISO 31010:2010 [3, 4]. ISO 9001:2015 does not require a risk management process, whereas ISO 14001 does. If the management system is integrated, then at the level of this system there must be a risk management process that will apply to both quality and environment. The same requirement is imposed by ISO 45001:2018 [5].
The integration of risk management at the level of organizational processes and practices ensures the following advantages:
increasing effectiveness and efficiency in achieving objectives;
increasing confidence and taking appropriate responsibility for the effective application of risk and opportunity thinking;
creating a safe working environment for pharmacists and patients;
control of all processes within the organization.
The great advantage of the ISO 9001:2015 standard is represented by the description of all the stages of applying a risk management process, namely thinking based on risk and opportunity [6]. Omitting one or more stages in this process leads us to a formal approach that has nothing to do with the future, nor with a proactive management system that ensures the prevention of the materialization of risks and their negative effects, in other words, do we prevent it instead of evaluating managers by results, not by effort [7].
Despite a significant amount of research in the field of risk management, many important issues have yet to be resolved. Until now, in the Republic of Moldova, there is no consensus regarding the essence and content of risk from pharmaceutical activity, the criteria, and indicators for risk assessment are not justified, and there is no scientifically based classification of risk factors, especially the risk factors present in community pharmacies.
Another problem is to achieve a common approach to the application of the concept of risk management among different stakeholders, because each stakeholder perceives the possibility of the occurrence of other harmful aspects perceives differently the probability of occurrence of each harmful phenomenon and can attribute different impact to each such factor of risk. In the pharmaceutical field, despite the diversity of stakeholders, from patients and doctors to the state and industry, patient protection through quality risk management must be considered of utmost importance [8].
This research represents a scientific approach to the application of modern risk management methods in community pharmacy and may be of use to pharmaceutical specialists, community pharmacists, and especially pharmacist managers.
The purpose of the study was to research and demonstrate the usefulness of modern risk management strategies and to evaluate the impact of risk management in the provision of quality services by community pharmacies.
Material and methods
Risk management provides scientific and practical support for decision-making. It provides documented and reproducible methods for implementing the quality risk management process based on current knowledge of risk probability, impact, and exposure [9].
The purpose of risk management is not to avoid risks at all costs, reducing risks to zero, in most cases being impossible and rarely can be done at reasonable cost. Therefore, accepting a certain degree of risk is sometimes necessary within the organization. We are talking here about „risk appetite” which according to ISO 31000 (risk management standard) is „the amount and type of risk that an organization is prepared to pursue, retain or assume” [10].
Risk can be identified and assessed using risk management tools. Risk identification and assessment methods can be used in combination with statistical tools [11]. Combined use provides the flexibility that can facilitate the application of quality risk management principles [12]. By applying modern risk management methods, this research presents the risks that may arise during the exercise of pharmaceutical activity within the community pharmacy. The modern risk management methods applied in this research are the following: the CNAM (Conservatoire national des arts et métiers) method combined with the Delphi Technique, the Ishikawa Diagram, the analysis method of MAE experts in risk assessment, the Risk Matrix and the risk ranking method.
The principle of the CNAM method consists in identifying all the risks that can influence the activity of employees [13, 14]. A group of 10 pharmacists was given a checklist, which was discussed in 3 rounds, applying the Delphi Technique. The checklist contains a series of risks and a question that must be answered with YES or NO: „Can the presented risks negatively influence the activity of the pharmacist and increase the incidence of medication errors?”. If the treated problem indicates the existence of a new risk, regardless of whether it is considered important or not, real or assumed, it is entered in a special form „Identification of new risks” [15].
After all the check-lists were completed, the identification form was resumed, regroupings were made and it was completed with the risks that were not initially identified during the investigation. Thus, 56 potential risks that can cause medication errors in community pharmacies were identified.
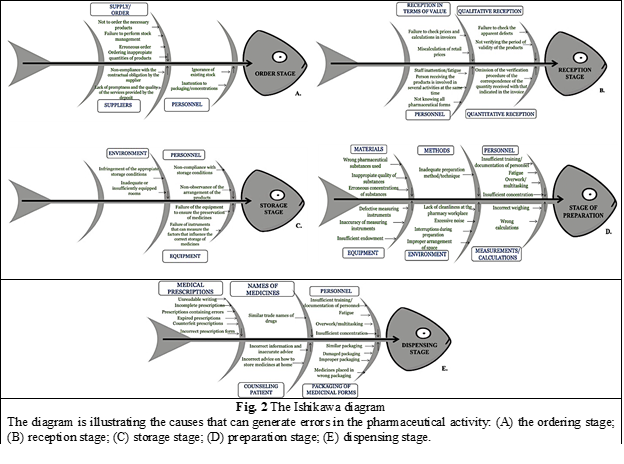
To sort all identified risks into categories, the Ishikawa diagram was used, this being an important method used in quality management [16]. The Ishikawa diagram was applied in this research to graphically illustrate the causes that can generate errors in the preparation, ordering, receiving, storage, and dispensing of medicines.
To assess the level of probability and impact of each identified risk, the expert analysis method (MAE) in risk assessment was used [17]. The method was based on surveying several independent experts to assess the level of risk.
Assessing the probability of occurrence of risks involves determining or estimating a probability. A possible method of estimating the probability of the materialization of the risk is the calculation of the frequency of the materialization of some risks in the past, table 1.
The assessment of the impact on the objectives/activities in case of materialization of risks was carried out according to the Impact Assessment Scale, presented in table 2.
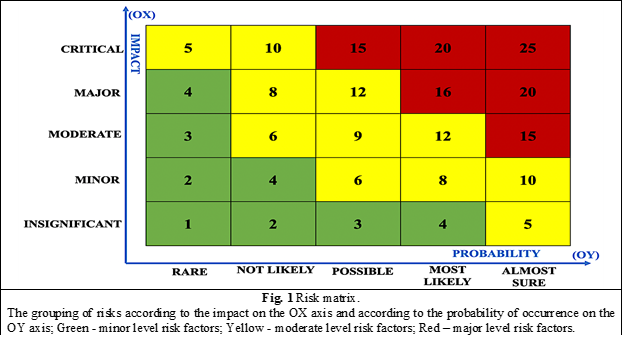
Afterwards follows the stage in which the risk exposure (risk factor), which is a combination of probability and impact, being a two-dimensional, matrix-type indicator [18], is set up. It can be represented in several forms, depending on the model adopted to estimate the probability and impact of materializing the risk. In this research we used the Risk Matrix, for a graphical visualization of the results. Based on the calculated risk factor, the risks can be ranked. The ranking of risks is used to establish priorities in order to plan preventive actions. The risk that obtained the highest value of the risk factor is entered first on the document used for ranking [19].
The risk matrix (figure 1), allows a qualitative assessment and facilitates the grouping of risks according to the impact on the OX axis and according to the probability of occurrence on the OY axis. The matrix graphically highlights minor risks, moderate risks and major risks [20].
The interaction between the level of probability and the impact associated with a risk generates the following priority categories.
major risks (Priority 1) – require attention to address/implement urgent and appropriate prevention/control measures;
moderate risks (Priority 2) – can be monitored or controlled, by increasing the effectiveness of existing measures or, as the case may be, establishing additional prevention / control measures;
minor risks (Priority 3) – can be tolerated and will be considered inherent in the activities against which additional prevention/control measures should not be established, but only the application of the existing ones [21].
According to the results of the priority categories, risk response actions can be developed according to the following classification:
risk acceptance (tolerance) – recommended for low exposure risks and does not require risk control measures to be taken;
risk monitoring – acceptance of the risk, provided that it is kept under permanent supervision. The monitored parameter is the probability, because the monitoring strategy is applied in the case of risks with a significant impact, but with a low probability of occurrence. Monitoring implies a postponement of taking control measures, until the moment when the circumstances determine an increase in the probability of occurrence of the risks subject to this type of treatment;
risk avoidance (elimination) – elimination of the activities that generate the risks;
risk transfer (outsourcing) – entrusting risk management to a third party that has the necessary capacity to manage this risk;
treating (mitigating) risks – implementing managerial internal control tools/measures to keep risks within acceptable (tolerable) limits [22, 23].
Results
By applying the CNAM Method combined with the Delphi Technique, a number of 56 potential risks, that can influence the occurrence of medication errors, were identified. Because of the multitude of causes that can lead to errors, the potential risks of errors were sorted by applying the 5-step Ishikawa Diagram, figure 2.
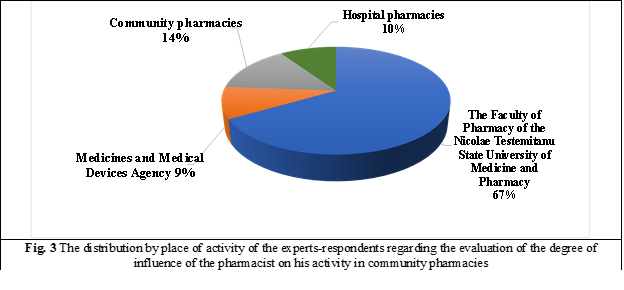
The next stage of risk analysis is the assessment of the degree of its influence on the pharmacist's activity in community pharmacies. The estimation of the probability of materialization and the impact was carried out by the expert analysis method (MAE) in risk assessment. The concrete criteria that served as a basis for the selection of experts were: place of work, position held, work experience, studies, scientific title, qualification category, approximate number of scientific articles held in the field of pharmaceutical system activity. In the pre-selection process, 21 specialists were trained, 14 of whom were the staff of the Faculty of Pharmacy of Nicolae Testemitanu State University of Medicine and Pharmacy, 2 experts were selected from the Medicines and Medical Devices Agency, 3 from community pharmacies, and 2 experts from hospital pharmacies, figure 3.
The results of the risk assessment by experts as well as the amount of risk exposure (risk factor) at various stages of drug circulation are presented in tables 3-7. Risk factors with major values are marked in red, and those with moderate values - with yellow corresponding to the risk matrix.
The value of each risk factor determines the levels of intervention priorities for risk removal, described in Table 8.
Discussion
In total, the risk factor was calculated for a number of 56 potential risk factors that can cause medication errors, categorized in 5 stages, of which 8 for the activity of ordering medicines (grouped into 3 categories: order, personnel, suppliers), 8 potential risks for the product reception activity (grouped into 4 categories: qualitative reception, reception in terms of value, personnel, quantitative reception), 6 risks for the activity of storing products in the pharmacy (grouped into 3 categories: personnel, environment, equipment), 17 causes of errors for the drug preparation activity (grouped into 6 categories: personnel, methods, materials, measurements/calculations, environment, equipment) and 17 causes of errors for the activity of dispensing medicinal products (grouped into 5 categories: personnel, names of medicines, packaging of medicinal forms, medical prescriptions, counseling of patients).
Based on the calculated risk factor value, the risks were ordered into major level risks (Intervention Priority 1), moderate level risks (Intervention Priority 2) and minor level risks (Intervention Priority 3). Thus, for the stage of ordering the medicines, 7 moderate risks (priority 2) and 1 major risk (priority 1) were detected, for the stage of receiving the medicines there are 7 moderate risks (priority 2) and 1 major risk (priority 1), for the drug storage stage there are 6 moderate risks (priority 2), for the drug preparation stage 14 moderate risks (priority 1) and 3 major risks (priority 1) were calculated and the drug release stage contains 9 moderate risks ( priority 2) and most major risks (priority 1) - 8. In total, in the 5 stages analyzed, no minor level risk (priority 3), 43 moderate level risks (priority 2) and 13 major level risks (priority 1) were detected.
All moderate and major risks will be included in the „Risk Assessment Sheet”, indicating: Preventive actions used; Risk evaluation; Planned actions to reduce risk, in descending order, the first being the risks that obtained the highest value of the risk factor.
Following the research carried out, the following activities with a major level of risk (priority 1) were highlighted, for which urgent measures to minimize the impact are required: illegible writing in medical prescriptions (15.5), fatigue of pharmaceutical staff (15.48), overwork/multitasking (14.99), prescriptions containing errors (14.26), insufficient staff knowledge (13.81 ), ordering inappropriate quantities of products in pharmacies (13.59), incomplete prescriptions (12.86), similar drug packaging (12.63) and insufficient staff concentration (12.18).
Conclusions
Risk management, as a component of an effective quality system, is an activity that integrates the identification, analysis, assessment of risk and the development of their management strategies. By applying the CNAM Method and the Delphi Technique, 56 potential risks that can cause medication errors were identified, which were later categorized using the Ishikawa Diagram in 5 stages, of which 8 for the activity of ordering medicines (grouped into 3 categories: order, personnel, suppliers), 8 potential risks for the product reception activity (grouped into 4 categories: qualitative reception, reception in terms of value, personnel, quantitative reception), 6 risks for the activity of storing products in the pharmacy (grouped into 3 categories: personnel, environment, equipment), 17 causes of errors for the drug preparation activity (grouped into 6 categories: personnel, methods, materials, measurements/calculations, environment, equipment) and 17 causes of errors for the activity of dispensing medicinal products (grouped into 5 categories: personnel, names of medicines, packaging of medicinal forms, medical prescriptions, counseling of patients).
All the risks that can generate medication errors in community pharmacies were researched through the expert analysis method (MAE) in risk assessment and values of the probability of occurrence of risks and the impact of the result were obtained for each risk. The potential risks of error were graphically represented using the Risk Matrix and then ranked to be entered in the Risk Assessment Sheet. Thus, the following activities were highlighted with a major high level of risk (priority 1), for which urgent measures to minimize the impact are required: illegible writing in medical prescriptions (15.5), fatigue of pharmaceutical staff (15.48), overwork/multitasking (14.99), prescriptions containing errors (14.26), insufficient staff knowledge (13.81 ), ordering inappropriate quantities of products in pharmacies (13.59), incomplete prescriptions (12.86), similar drug packaging (12.63) and insufficient staff concentration (12.18).
The results of this work constitute the basis for future research with proposals to reduce the risks of errors in community pharmacy.
In this research, risk management methods were used to optimize pharmacy activities and increase their performance.
In order to ensure the continuity of the chain of ordering-receiving-storage-preparation-dispensing the medicines, and to minimize or prevent the risks that can generate medication errors, we propose to follow the steps:
Make risk management a key element;
Identify risks using a holistic approach;
Set up the risk assessment tools;
Ensure that risk management is an ongoing activity, not a one-off action;
Carry out periodic checks on the actions taken;
Maintain appropriate standards for procedures, duties, responsibilities, etc.
This study presents practical utility, the research results can be applied by pharmacies, and following the key steps mentioned above will contribute to improving performance and modernizing pharmaceutical management.
Competing interests
None declared
Author’s contribution
NCB conceived the study, study design, performed the experimental procedures, data collection, analysis and interpretation, drafting the manuscript. NCB, MB, participated in the study design, data collection and helped to draft the manuscript, approved the final version of the manuscript, ready for publication. NCB, SA, MB performed the data collection, helped to draft the manuscript, manuscript preparation. SA, MB participated in the design of the study, revised the manuscript critically, providing important intellectual input, approved the final version of the manuscript.
Authors’ ORCID IDs
Nicoleta Cheptanari-Birta - https://orcid.org/0000-0002-1331-1161
Stela Adauji - https://orcid.org/0000-0002-5027-4144
Mihail Brumărel - https://orcid.org/0000-0003-1126-9884
References
Александров A. Воспитание привычки к управлению рисками для качества. Фармацевтическая отрасль, 2015; 4 (51): 112–114.
Ismael O., Ahmed M. Using quality risk management in pharmaceutical industries: A case study. Quality - Access to Success, 2020; 21(178): 106–113.
ISO 14001:2015. Environmental management systems- Requirements with guidance for use.ISO.org. https://www.iso.org/standard/60857.html.
ISO 9001:2015. Quality management systems - Requirements. ISO.org. https://www.iso.org/standard/62085.html].
ISO 31000:2018. Risk management – Guidelines. ISO.org. https://www.iso.org/standard/65694.html.
ISO 45001:2018. Occupational health and safety management systems - Requirements with guidance for use. ISO.org. https://www.iso.org/standard/63787.html.
Baker N. Quality Risk Management (QRM). Pharmaceutical Quality by Design: A Practical Approach, 2017; 1(2): 11–45. doi: 10.1002/9781118895238.ch2.
Cheptanari-Birta N., Brumărel M., Safta V., Spinei L., Adauji S. The analysis of prescriptions and distribution of medicines in the prevention of medication errors in community pharmacies. Farmacia, 2022; 70 (4): 760-766. https://farmaciajournal.com/wp-content/uploads/art-25-Cheptanari-Birta_….
Claycamp H. Probability Concepts in Quality Risk Management. Journal of Pharmaceutical Science and Technology, 2012; 66(1): 78–89. doi: 10.5731/pdajpst.2012.00801
Cheptanari-Birta N., Brumarel M., Safta V., Adauji S. Discipline of pharmaceutical risk management - integrated component in the training of specific professional competences of pharmacists. Матеріали VІІ міжнародної науково-практичної конференції, присвяченої 10-річчю кафедри соціальної фармації, 2021; 8(1): 199-208.
Sharma A., Jeyaprakash R., Chandra A. Impact of quality risk management process in pharmaceutical industry to curtail the non-conformity. International Journal of Pharmaceutical Quality Assurance, 2020; 11(1): 179-185. doi: 10.25258/ijpqa.11.1.28.
Balmoş M., Lazǎr M. Quality risk Management in the Pharmaceutical industry. Quality - Access to Success, 2013; 14(136): 73–75.
Abu Hagar R., El-Dahiyat F., El Refae G. Risk management in community pharmacy practice in Abu Dhabi Region: a cross-sectional study. Journal of Pharmaceutical Health Services Research, 2020; 11(3): 275–285. doi: 10.1111/jphs.12364.
Charoo N., Ali A. Quality risk management in pharmaceutical development. Drug Development and Industrial Pharmacy, 2013; 1(49): 947-960. doi: 10.3109/03639045.2012.699065.
Claycamp H., Rahaman F., Urban J. The reliability-quality relationship for quality systems and quality risk management. Journal of Pharmaceutical Science and Technology, 2012; 66 (6): 512-517. doi: 10.5731/pdajpst.2012.00888.
Kumar N., Jha A. Quality risk management during pharmaceutical ‘good distribution practices’ – A plausible solution. Bulletin of Faculty of Pharmacy, Cairo University, 2018; 56(1): 18–25. Available at the address: doi: 10.1016/j.bfopcu.2017.12.002.
Mandhare T., Khuspe P., Nangare P. et al. Quality Risk Management: A Review. American Journal of PharmTech Research, 2018; 8(2): 56–86.
Kashirina A., Aladysheva Z., Pyatigorskaya N. et al. Analysis of industrial practice of drug quality risk management in Russian pharmaceutical enterprises. Farmatsiya i Farmakologiya, 2021; 8(5): 362–376. doi: 10.19163/2307-9266-2020-8-5-362-376.
Cheptanari-Birta N. Management of pharmaceutical risk factors – warranty of patient’s safety. Moldovan Medical Journal, 2020; 63(2): 49-53. doi: 10.5281/zenodo.3866023.
Olechowski A., Oehmen J., Seering W. The professionalization of risk management: What role can the ISO 31000 risk management principles play? International Journal of Project Management, 2016; 34(8): 1568–1578. doi: 10.1016/j.ijproman.2016.08.002.
Prashant K. Risk analysis and risk management in pharmaceutical industry. International Journal of Pharma World Research, 2010; 1(1): 56-59.
Prashar A., Aggarwal S. Modeling enablers of supply chain quality risk management: a grey-DEMATEL approach. TQM Journal, 2020; 32(5): 1059–1076. doi: 10.1108/TQM-05-2019-0132].
Suprin M., Chow A., Pillwein M. Quality Risk Management Framework: Guidance for Successful Implementation of Risk Management in Clinical Development. Therapeutic Innovation and Regulatory Science, 2019; 53(1): 36–44. doi: 10.1177/2168479018817752.
Cheptanari-Bîrta N. General provisions on medication errors committed by pharmacists. Moldovan Medical Journal, 2020; 63(1): 61-65. doi: 10.5281/zenodo.3685669.
Table 1. Risk probability assessment scale. | |||
PROBABILITY | 1 | Rare | It is very unlikely to happen over a long period of time (3 - 5 years); it has not happened so far. |
2 | Not likely | It is unlikely to happen over a long period of time (3 - 5 years); it has happened very few times so far. | |
3 | Possible | It is likely to happen over a medium period of time (1-3 years); it has happened a few times in the last 3 years. | |
4 | Most likely | It is likely to occur over a short period of time (< 1 year); it has happened a few times in the last year. | |
5 | Almost sure | It is very likely to happen over a short period of time (< 1 year); it has happened many times in the last year. | |
Table 2. Risk Impact Assessment scale [17]. | |||
IMPACT | 1 | Insignificant | With very low impact on activities and the achievement of objectives and/or no financial impact. |
2 | Minor | With low impact on activities and achievement of objectives and/or with very low financial impact. | |
3 | Moderate | With medium impact on activities and the achievement of objectives and/or with medium financial impact. | |
4 | Major | With major impact on the activities and achievement of objectives and/or with major financial impact. | |
5 | Critical | With a significant impact on activities and the achievement of objectives and/or with a significant financial impact. | |
Table 3. The results of the application of the expert analysis method (MAE) in risk assessment of the MEDICINE ORDERING STAGE | |||
|---|---|---|---|
Activities potential risks/hazards | Risk assessment | ||
Probability (1-5) | Impact (1-5) | The value of the risk factor | |
Supply/Order | |||
| Non-ordering the necessary products | 2,75 | 3,81 | 10,47 |
| Incorrect performing of stock management | 3,00 | 3,87 | 11,62 |
| Erroneous order (wrong products, from another manufacturer, another supplier) | 2,50 | 2,75 | 6,87 |
| Ordering inappropiate quantities of products (too large or too small) in relation to the needs of the pharmacy | 3,75 | 3,62 | 13,59 |
Personnel | |||
| Ignorance of the existing stock by the pharmacist | 2,50 | 3,18 | 7,96 |
| Inattention to the packaging, the differences in concentrations of products etc. | 2,50 | 3,75 | 9,37 |
Suppliers | |||
| Non-compliance with contractual obligations by the supplier | 2,62 | 3,25 | 8,51 |
| Lack of promptness and the quality of the services provided by the deposit | 2,00 | 3,00 | 6,00 |
| Note: Red – major level risk factors; Yellow - moderate level risk factors. | |||
Table 4. The results of the application of the expert analysis method (MAE) in risk assessment of the STAGE ON RECEPTION | |||
|---|---|---|---|
Activities potential risks/hazards | Risk assessment | ||
Probability (1-5) | Impact (1-5) | The value of the risk factor | |
Qualitative reception | |||
| Failure to check the apparent defects (ex. broken packaging, damaged blisters etc.) | 2,12 | 3,43 | 7,28 |
| Not verifying the period of validity of the products | 2,75 | 4,12 | 11,33 |
Reception in terms of value | |||
| Failure to check prices and calculations in invoices | 2,00 | 3,37 | 6,75 |
| Miscalculation of retail prices | 1,25 | 3,25 | 4,06 |
Personnel | |||
| Staff inattention/fatigue | 3,12 | 3,75 | 11,7 |
| Not knowing all pharmaceutical forms | 2,75 | 3,75 | 10,31 |
| Person receiving the products is involved in several activities at the same time | 3,62 | 3,87 | 14,00 |
Quantitative reception | |||
| Omission of the verification procedure of the correspondence of the quantity received with that indicated in the invoice | 2,50 | 3,62 | 9,06 |
| Note: Red – major level risk factors; Yellow - moderate level risk factors. | |||
Table 5. The results of the application of the expert analysis method (MAE) in risk assessment of the MEDICINE STORAGE STAGE | |||
|---|---|---|---|
Activities potential risks/hazards | Risk assessment | ||
Probability (1-5) | Impact (1-5) | The value of the risk factor | |
Personnel | |||
| Non-compliance with storage conditions | 3,00 | 3,62 | 10,87 |
Non-observance of the arrangement of the products | 3,25 | 3,00 | 9,75 |
Environment | |||
| Infringement of the appropriate storage conditions taking into account the environmental factors that can affect the preservation of drugs (temperature, humidity, light, atmospheric air etc.) | 2,87 | 3,75 | 10,76 |
| Inadequate or insufficiently equipped rooms (lack of cupboards, shelves, safes) | 2,25 | 3,62 | 8,15 |
Equipment | |||
| Failure of equipment for ensuring the necessary preservation conditions (e.g. pharmacy display refrigerators etc.) | 2,00 | 3,18 | 6,36 |
| Failure of appropriate instruments to measure factors that may affect storage and preservation (e.g. thermometers, hygrometers etc.) | 2,12 | 2,62 | 5,55 |
Personnel | |||
| Insufficient training/ documentation of personnel | 3,25 | 4,25 | 13,81 |
| Fatigue | 3,87 | 4,00 | 15,48 |
| Overwork/multitasking | 3,62 | 3,81 | 13,80 |
| Insufficient concentration (monotonous work) | 2,62 | 3,37 | 8,82 |
Methods | |||
| Inadequate preparation method/ technique | 2,50 | 3,50 | 8,75 |
Materials | |||
| Wrong pharmaceutical substances used | 2,12 | 4,12 | 8,74 |
| Inappropriate quality of substances (expired, inadequately stored, improperly packed etc.) | 1,87 | 3,81 | 7,12 |
| Erroneous concentrations of substances | 2,12 | 4,12 | 8,74 |
Measurements, calculations | |||
| Incorrect weighing | 2,50 | 3,93 | 9,84 |
| Wrong calculations | 2,62 | 3,93 | 10,31 |
Environment | |||
| Lack of cleanliness at the pharmacy workplace | 1,62 | 2,62 | 4,25 |
| Excessive noise | 2,62 | 2,87 | 7,53 |
| Interruptions during preparation | 2,25 | 3,25 | 7,31 |
| Improper arrangement of the space | 2,00 | 3,00 | 6,00 |
Equipment | |||
| Defective measuring instruments (balances) | 2,12 | 4,06 | 8,61 |
| Inaccuracy of measuring instruments | 2,00 | 4,12 | 8,25 |
| Insufficient equipment (cabinets, utensils, raw materials) | 2,37 | 3,56 | 8,44 |
| Note: Red – major level risk factors; Yellow - moderate level risk factors. | |||
Table 6. The results of the application of the expert analysis method (MAE) in risk assessment of the DRUG PREPARATION STAGE | ||||
Activities potential risks/hazards | Risk assessment | |||
Probability (1-5) | Impact (1-5) | The value of the risk factor | ||
Personnel | ||||
| Insufficient training/ documentation of personnel | 3,25 | 4,25 | 13,81 | |
| Fatigue | 3,87 | 4,00 | 15,48 | |
| Overwork/multitasking | 3,62 | 3,81 | 13,80 | |
| Insufficient concentration (monotonous work) | 2,62 | 3,37 | 8,82 | |
Methods | ||||
| Inadequate preparation method/ technique | 2,50 | 3,50 | 8,75 | |
Materials | ||||
| Wrong pharmaceutical substances used | 2,12 | 4,12 | 8,74 | |
| Inappropriate quality of substances (expired, inadequately stored, improperly packed etc.) | 1,87 | 3,81 | 7,12 | |
| Erroneous concentrations of substances | 2,12 | 4,12 | 8,74 | |
Measurements, calculations | ||||
| Incorrect weighing | 2,50 | 3,93 | 9,84 | |
| Wrong calculations | 2,62 | 3,93 | 10,31 | |
Environment | ||||
| Lack of cleanliness at the pharmacy workplace | 1,62 | 2,62 | 4,25 | |
| Excessive noise | 2,62 | 2,87 | 7,53 | |
| Interruptions during preparation | 2,25 | 3,25 | 7,31 | |
| Improper arrangement of the space | 2,00 | 3,00 | 6,00 | |
Equipment | ||||
| Defective measuring instruments (balances) | 2,12 | 4,06 | 8,61 | |
| Inaccuracy of measuring instruments | 2,00 | 4,12 | 8,25 | |
| Insufficient equipment (cabinets, utensils, raw materials) | 2,37 | 3,56 | 8,44 | |
| Note: Red – major level risk factors; Yellow - moderate level risk factors. | ||||
Table 7. The results of the application of the expert analysis method (MAE) in risk assessment of the PRODUCTS DISPENSING STAGE | |||
|---|---|---|---|
Activities potential risks/hazards | Risk assessment | ||
Probability (1-5) | Impact (1-5) | The value of the risk factor | |
Personnel | |||
| Insufficient training/ documentation of personnel | 3,12 | 4,31 | 13,44 |
| Fatigue | 3,50 | 3,87 | 13,56 |
| Overwork/multitasking | 3,87 | 3,87 | 14,99 |
| Insufficient concentration (monotonous work) | 3,25 | 3,75 | 12,18 |
Names of medicines | |||
| Similar trade names of drugs | 2,87 | 3,50 | 10,04 |
The packaging of medicinal forms | |||
| Similar packaging | 3,37 | 3,75 | 12,63 |
| Damaged packaging | 2,50 | 3,50 | 8,75 |
| Improper packaging that does not allow for proper storage of medicines | 2,25 | 3,75 | 8,43 |
| Medicines placed in wrong packaging | 1,50 | 3,75 | 5,62 |
Medical prescriptions | |||
| Unreadable writing | 4,00 | 3,87 | 15,5 |
| Incomplete prescriptions | 3,43 | 3,75 | 12,86 |
| Prescriptions containing correctable/uncorrectable errors | 3,68 | 3,87 | 14,26 |
| Expired prescriptions | 3,25 | 3,50 | 11,37 |
| Counterfeit prescriptions | 2,62 | 4,50 | 11,79 |
| Incorrect prescription form | 2,75 | 3,12 | 8,59 |
Counseling patients | |||
| Incorrect information and inaccurate advice | 2,62 | 4,25 | 11,13 |
| Incorrect advice on how to store medicines at home | 2,62 | 3,87 | 10,15 |
| Note: Red – major level risk factors; Yellow - moderate level risk factors; | |||
| Table 8. The priority of the intervention against the risk depending on the value of the risk factor. | |||
|---|---|---|---|
Level of risk | Intervention priority | Response to risk | |
Major level of risk The value of the risk factor (13-25) | Intervention priority 1 | Avoiding, transferring or dealing with risks | These risks are not tolerable. The head of the entity must focus on the urgent adoption and implementation of appropriate prevention and control measures. |
Moderate level of risk The value of the risk factor (5-12) | Intervention priority 2 | Risk monitoring Dealing with risks | The manager could manage the risks by streamlining and effectively applying existing measures or, as necessary, by adopting additional prevention and control measures. |
Minor level of risk The value of the risk factor (1-4) | Intervention priority 3 | Risk acceptance (tolerance). Risk monitoring | The risk can be tolerated. The manager must effectively apply existing prevention and control measures. New measures are needed if possible without significant additional resources or efforts. |
| Note: Red – major level risk factors; Yellow - moderate level risk factors; Green - minor level risk factors | |||