Introduction
The umbilical cord (UC) is essential for fetal survival, facilitating necessary exchanges between the mother and fetus. Umbilical cord pathologies, such as true knots, hypercoiling, velamentous insertion, cord entanglement, and cord prolapse, are often associated with fetal hypoxia and can significantly impact perinatal outcomes, leading to complications and even fetal death [1-3]. Recent studies show that umbilical cord abnormalities may be associated with an increased risk of preterm birth and the need for intensive neonatal care, highlighting the importance of identifying and monitoring these pathologies during pregnancy [4]. Placental and umbilical cord examinations in cases of fetal death indicate that a significant portion of fetal deaths can be attributed to cord pathologies [5]. In this context, understanding the underlying causes of umbilical cord pathologies can aid clinical management by identifying etiopathogenesis and improving perinatal outcomes [6, 7].
Nonetheless, existing research provides only limited insight into predictive factors and effective management strategies for the risks associated with umbilical cord pathology, as well as the complex relationships between umbilical cord structure, obstetric outcomes, and perinatal health. Despite ongoing research, there is still a lack of comprehensive understanding in the literature regarding these predictive factors and management strategies. Additionally, the complex interactions between the structure of the umbilical cord, obstetric outcomes, and perinatal health remain insufficiently explored. This research could enable a deeper understanding of pathological mechanisms and support the development of strategies that could improve prenatal care and reduce the incidence of complications associated with umbilical cord pathologies.
The aim of the study is to determine the clinical and evolutionary characteristics of pregnancies and deliveries in patients with umbilical cord pathology.
Material and methods
The study was conducted at the Department of Obstetrics and Gynecology, Nicolae Testemițanu State University of Medicine and Pharmacy, in the Republic of Moldova. A total of 190 patients participated, divided into two groups: L1 – 95 patients with umbilical cord (UC) abnormalities and L0 – 95 with normal UC. This prospective cohort study included data on demographics, medical history, pregnancy progression, and perinatal outcomes. The data were analyzed using statistical and mathematical methods with SPSS 23.0, SAS 9.4, and Microsoft Office Excel 2016. These tools enabled the calculation of rates, mean values, and proportion indicators, as well as the determination of statistical significance at a 95% confidence level. The Pearson chi-square test (χ2) was applied to discrete variables, and odds ratios (OR) with confidence intervals (CI) were calculated. The study aimed to investigate the relationship between umbilical cord pathology and pregnancy complications and delivery outcomes.
Results
Most women in both groups were between 20 and 34 years old (mean age 29.09±4.85 years in first group vs. 27.86±4.36 years in the second one, p>0.05), with a coefficient of variation of 15.66% for L0 and 16.66% for L1. The assessment of socioeconomic status between the groups did not show statistically significant differences (p>0.05).
In addition to age and socioeconomic factors, we analyzed the determinants of umbilical cord pathology that influence its development (Table 1). The evaluation of harmful workplace factors for patients in group L1 revealed that these factors were recorded more frequently. However, only psychological and emotional stress showed a correlation with umbilical cord pathology (CO), with χ²1df = 5.9047, Cramer's V = 0.21, and p=0.01.
Table 1. Determinant factors for umbilical cord pathology | |||||
Factor | Study group (n=95) | Control group (n=95) | OR | 95% CI | p |
Psychological and Emotional Stress at Work | 26 | 14 | 2,5536 | 1,1861-5,4976 | 0,01 |
Harmful Habit (Smoking) | 10 | 3 | 1,0824 | 1,0012-1,1701 | 0,04 |
Primiparity | 49 | 28 |
|
| 0,005 |
Umbilical Cord Pathology in Previous Pregnancies | 34 | 20 |
|
| <0,0001 |
Infertility | 9 | 1 |
|
| 0,02 |
Urinary Tract Disorders | 40 | 25 | 1,2727 | 1,0323-1,5692 | 0,02 |
Polyhydramnios | 11 | 1 | 2,1190 | 2,0375-2,2070 | 0,002 |
Urgent Cesarean Section | 11 | 2 | 5,0769 | 1,9287-27,7546 | 0,04 |
Note: OR – odds ratio; CI – confidence interval. | |||||
The analysis of behavioral risk factors found a high incidence of harmful habits in patients with umbilical cord pathology, with smoking being a significant determinant (χ²1df=4.0461, Cramer's V=0.2; p=0.04). Regarding parity, the samples were heterogeneous, with primiparity emerging as a key factor that correlated with the risk of umbilical cord pathology (χ²2df=10.2928, Cramer's V=0.23; p=0.005). An indirect Pearson correlation (rxy=-0.33; p=0.001) was established between the number of spontaneous abortions in the patient's history and the number of umbilical vessels observed on prenatal ultrasound in the current pregnancy, suggesting a risk of vascular anomalies in the umbilical cord for women with a history of spontaneous abortions.
The study's data on patients' personal medical history revealed a relatively high percentage of urinary tract disorders (chronic pyelonephritis, bacteriuria, chronic cystitis, hydronephrosis, nephroptosis, renal colic, pyeloectasia, nephrolithiasis, double kidney), which were more prevalent in the L1 group, with 40 (42.11%) cases compared to 25 (26.32%) cases in the L0 group (χ²1df=5.2615, Cramer's V=0.2; p=0.02).
During the study, it was found that one in every three to four women in L1 had at least one episode of imminent pregnancy termination, with such incidents occurring more frequently around the 27-28-week gestation mark (p<0.05). Additionally, polyhydramnios was detected 10 times more frequently in L1 compared to the control group, confirming a significant link between polyhydramnios and umbilical cord pathology (p<0.05).
As for fetal growth restriction (FGR), it was observed only in the L1 group (χ²1df=5.1351, Cramer's V=0.2; p=0.02), with a predictive model showing AUC=0,8679 (Fig. 1). It is important to emphasize that the role of umbilical cord pathology should not be overlooked, as such conditions often lead to profound changes in the fetoplacental complex, resulting in fetal distress.
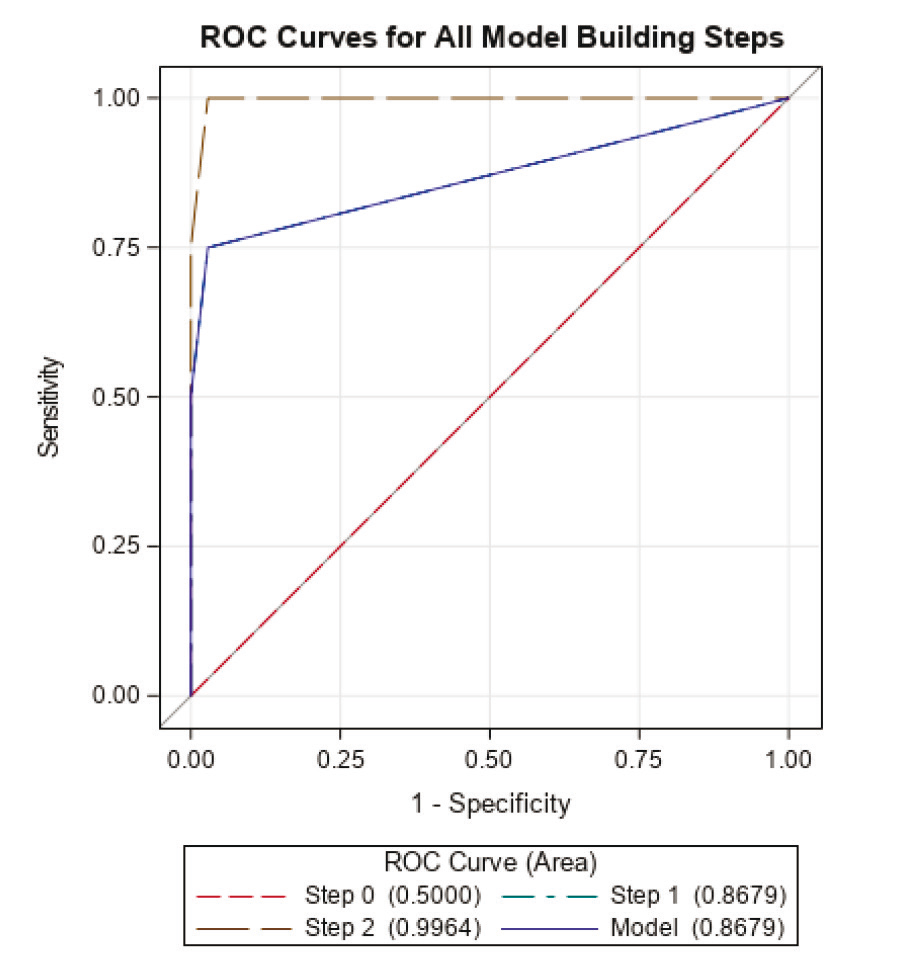 |
Fig. 1 The ROC curve of the predictive model for the probability of developing fetal growth restriction in children with umbilical cord pathology, based on perinatal morbidity |
Following the evaluation of the duration of the anhydramnios period, it was found that in patients with umbilical cord pathology, this period was prolonged – up to 109 hours and 45 minutes, with a mean of 9.17±17.76 hours (95% CI 5.11-13.23). In the L0 group, the duration ranged from 5 minutes to 18 hours and 15 minutes, with a mean of 3.69±3.65 hours (95% CI 2.88-4.49), with p<0.05. The significant difference in the duration of the anhydramnios period between the compared groups is also clearly illustrated in Figure 2.
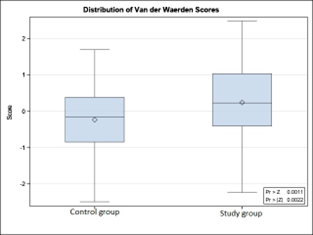 | 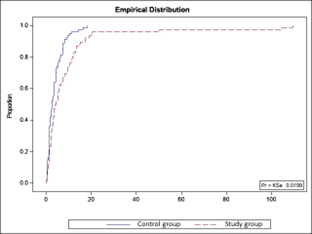 |
Fig. 2. Distribution of study groups by the duration of the anhydramnios period (hours/minutes). | |
In evaluating the delivery outcomes for the women in the study groups, it was found that vaginal delivery predominated: 81 (85.26%) cases in L0 vs. 71 (74.73%) in L1. Cesarean sections were performed more often in the L1 group: 24 (25.26%) cases vs. 14 (14.74%) in L0 (χ²1df=2.1934, Cramer's V=0.1; p=0.01), with urgent cesarean sections being more common: 11 (11.58%) in L1 vs. 2 (2.11%) in L0 (χ²1df=3.9100, Cramer's V=0.32; p=0.04). Fetal distress during the second stage of labor, resolved via vacuum extraction, occurred in 12 (12.63%) patients in L1 compared to 1 case in L0 (χ²1df=11.1143, Cramer's V=0.26; p=0.0009).
Postpartum hemorrhage (>500 ml) was more prevalent in patients with umbilical cord pathology: 31 (32.55%) cases compared to 16 (16.80%) cases in those with a normal umbilical cord (p<0.05).
The analysis of anamnestic and clinical features of the perinatal period in patients with umbilical cord pathology, compared to those without this pathology, highlights that the presence of this condition is associated with psychological and emotional stress, a higher number of spontaneous abortions, a history of imminent pregnancy termination, complicated somatic history (nephro-urinary conditions), polyhydramnios, and intrauterine fetal growth restriction. These factors should be carefully considered in the antenatal care and the clinical management of delivery.
Discussion
Umbilical cord pathology constitutes a major issue in contemporary obstetrics. Examining its determining factors allows for a detailed understanding of the pathogenesis of various umbilical abnormalities that negatively impact perinatal outcomes [4].
Our study data show that the age of the pregnant women ranged between 20 and 34 years, with no statistical differences between the two groups. Similar findings have been published by other authors, who confirmed that the age of the participants varied between 20 and 35 years and did not constitute a risk factor for the development of umbilical cord pathology [8, 9].
The data from our study also demonstrate that pathological conditions of the umbilical cord are more frequently observed in primiparas, serving as a determinant factor (χ²2df =10.2928, Cramer’s V=0.23; p=0.005). The extragenital medical history of the patients included in the study was compromised by the presence of 2-3 pathologies, with urinary tract conditions being the most prevalent [OR=1.2727; 95% CI 1.0323-1.5692; p=0.02]. Similar data were reported in a recent systematic review and meta-analysis, where these conditions were identified as risk factors for the development of umbilical abnormalities [10].
The evolution, clinical management, and complications during the current pregnancy were examined. It was found that one in three or four women in the study group had at least one episode of imminent pregnancy termination, most frequently occurring around 27-28 weeks of gestation (p<0.05). A relevant study by Meyer et al. (2020) confirms that women with umbilical cord pathology are at an increased risk of premature pregnancy termination. According to this study, there is a strong association between umbilical cord abnormalities and an increased risk of preterm contractions and premature rupture of membranes, particularly in the second half of pregnancy, between weeks 24 and 28 of gestation [11]. Similarly, Sanchez et al. (2021) observed in a cohort of pregnant women with umbilical cord pathology that the incidence of pregnancy loss and the risk of prematurity were significantly higher among those with structural abnormalities of the umbilical cord, such as cord knots or cord entanglements around the fetal neck. These pathologies can lead to impaired blood circulation through the umbilical vessels, thereby increasing the risk of placental insufficiency and, consequently, premature birth [12]. Furthermore, Zhang et al. (2023) reported that umbilical cord pathology is a significant predictor of prematurity, especially during the 27–28-week gestation period, when the fetus is particularly vulnerable to circulatory disturbances. This period represents a critical stage for fetal organ development, particularly for the respiratory system, and any complication affecting umbilical circulation can rapidly lead to severe perinatal risks, including preterm birth [13]. The study by Dubetskyi (2023) examined the structural and functional aspects of the umbilical cord in relation to pregnancy outcomes and placental characteristics. Four patient groups were analyzed postnatally: Wharton’s jelly edema (10 samples), velamentous cord insertion (10 samples), single umbilical artery (10 samples), and a control group with physiological pregnancies (10 samples). A combined presentation of umbilical cord characteristics (51.1%) was common, such as the absence of Wharton’s jelly alongside velamentous insertion or a "thin" cord with a single umbilical artery. Additional factors like maternal age, obesity, nicotine use, and history of IVF were associated with increased incidence of umbilical cord abnormalities, prematurity, preeclampsia, and placental dysfunction. Clinical complications included prematurity (24.4%), oligohydramnios (45.6%), preeclampsia (37.8%), placental dysfunction (44.4%), low birth weight (18.9%), neonatal pathologies (22.2%), and a higher rate of cesarean sections (32.2%) [14].
This finding underscores the importance of careful monitoring and evaluation of pregnant women, especially considering the perinatal risks more frequently encountered during the intermediate weeks of pregnancy.
Our study revealed a higher incidence of fetoplacental system pathologies in the group of patients with umbilical cord abnormalities, compared to those with a normal UC. These pathologies included polyhydramnios, oligohydramnios, fetal growth restriction, and intrauterine infection. Other studies have also highlighted that amniotic fluid acts as a protective factor, helping prevent compressions, as long as the liquid environment is sufficient and clear. Otherwise, it becomes a major risk factor for complications, leading to reduced blood flow in the UC [15].
FGR was diagnosed in cases of marginal and velamentous insertion, AOU, umbilical cord coiling, thin umbilical cord, hypocoiled, or torsioned cord. Notably, the research group recorded nearly twice as many cases of UC pathology in previous pregnancies (p<0.0001), placing these patients at higher risk for complications in their current pregnancies as well. Studies by Li et al. (2022) and Zhang et al. (2023) demonstrated that marginal and velamentous insertion of the umbilical cord, combined with twisting or hypocoiling, can impair placental function and negatively affect fetal development, leading to an increased risk of FGR and preterm birth. Additionally, umbilical cord wrapping around the fetal neck and disrupted circulation are significant contributors to complications associated with FGR and higher miscarriage risk [16, 17]. Acute fetal distress due to umbilical abnormalities required urgent delivery using obstetric forceps (p=0.0009). Research by Lavric I. et al. (2021) also emphasized that indications for forceps extraction included umbilical cord coiling (66%) and short umbilical cord (16%) [18].
Conclusions
The results of the study suggest that psychoemotional stress, smoking, primiparity, adverse obstetric history with spontaneous abortions, the presence of polyhydramnios, and fetal growth restriction in the current pregnancy represent significant risks for the presence of umbilical cord pathology. Early identification of these and other risk factors, along with careful monitoring of pregnancies and early antenatal diagnosis of umbilical cord pathology, are crucial for minimizing perinatal complications that may arise from this condition.
Competing interests
None declared.
Authors’ contributions
IuD proposed the study area, conceived the study design, and oversaw the main aspects of its implementation. AA participated in the study design, included subjects in the research, performed the statistical analysis of the collected data, and drafted the manuscript. HC critically reversed the manuscript. All authors critically reviewed the work and approved the final version of the manuscript.
Acknowledgements and funding
The study had no external funding.
Patient consent
Obtained.
Ethics approval
The Research Ethics Committee of the Nicolae Testemițanu State University of Medicine and Pharmacy approved the study - Minutes no. 95 from 21.06.2017.
Authors’ ORCID IDs
Iurie Dondiuc – https://orcid.org/0000-0003-2869-0819
Alina Alsatou – https://orcid.org/0000-0003-0126-2275
Hristiana Capros – https://orcid.org/0009-0002-1517-2342
References
Ma'ayeh M, McClennen E, Chamchad D, Geary M, Brest N, Gerson A. Hypercoiling of the umbilical cord in uncomplicated singleton pregnancies. J Perinat Med. 2018 Aug 28;46(6):593-598. doi: 10.1515/jpm-2017-0034.
Slack JC, Boyd TK. Fetal vascular malperfusion due to long and hypercoiled umbilical cords resulting in recurrent second trimester pregnancy loss: a case series and literature review. Pediatr Dev Pathol. 2021 Jan-Feb;24(1):12-18. doi: 10.1177/1093526620962061.
Mittal A, Nanda S, Sen J. Antenatal umbilical coiling index as a predictor of perinatal outcome. Arch Gynecol Obstet. 2015 Apr;291(4):763-8. doi: 10.1007/s00404-014-3456-5.
Hayes DJL, Warland J, Parast MM, Bendon RW, Hasegawa J, Banks J, Clapham L, Heazell AEP. Umbilical cord characteristics and their association with adverse pregnancy outcomes: a systematic review and meta-analysis. PLoS One. 2020 Sep 24;15(9):e0239630. doi: 10.1371/journal.pone.0239630.
Tonni G, Lituania M, Cecchi A, Carboni E, Resta S, Bonasoni MP, Ruano R. Umbilical cord diseases affecting obstetric and perinatal outcomes. Healthcare (Basel). 2023 Sep 27;11(19):2634. doi: 10.3390/healthcare11192634.
Redline RW. Classification of placental lesions. Am J Obstet Gynecol. 2015 Oct;213(4 Suppl):S21-8. doi: 10.1016/j.ajog.2015.05.056.
Hammad IA, Blue NR, Allshouse AA, Silver RM, Gibbins KJ, Page JM, Goldenberg RL, Reddy UM, Saade GR, Dudley DJ, Thorsten VR, Conway DL, Pinar H, Pysher TJ; NICHD Stillbirth Collaborative Research Network Group. Umbilical cord abnormalities and stillbirth. Obstet Gynecol. 2020 Mar;135(3):644-652. doi: 10.1097/AOG.0000000000003676.
Bhadrashetty N, Gomathy E. Association of umbilical cord abnormalities and nonreassuring fetal heart rate and its perinatal outcome. Int J Clin Obstet Gynaecol. 2021;5(2):28-32. doi: 10.33545/gynae.2021.v5.i2a.862.
Linde LE, Rasmussen S, Kessler J, Ebbing C. Extreme umbilical cord lengths, cord knot and entanglement: risk factors and risk of adverse outcomes, a population-based study. PLoS One. 2018 Mar 27;13(3):e0194814. doi: 10.1371/journal.pone.0194814.
Vasa R, Dimitrov R, Patel S. Nuchal cord at delivery and perinatal outcomes: single-center retrospective study, with emphasis on fetal acid-base balance. Pediatr Neonatol. 2018 Oct;59(5):439-447. doi: 10.1016/j.pedneo.2018.03.002.
Meyer SP, Brown JA, Williams MR. Umbilical cord pathologies and pregnancy outcomes: a review of recent evidence. J Matern Fetal Neonatal Med. 2020;33(14):2304-2310. doi: 10.1080/jmf.2020.1717684.
Sanchez RA, Martinez LD, Garcia MP. Risk of prematurity in pregnancies with umbilical cord abnormalities. Am J Obstet Gynecol. 2021;225(2):213-221. doi: 10.1016/ajog.2021.02.024.
Zhang Y, Li Z, Yang S, et al. The effect of umbilical cord abnormalities on preterm birth: a cohort study. Am J Obstet Gynecol. 2023;228(2):283-90. doi: 10.1016/j.ajog.2022.09.022.
Dubetskyi BI, Makarchuk OM, Zhurakivska OY, Rymarchuk MI, Andriets OA, Lenchuk TL, Delva KM, Piron-Dumitrascu M, Bakun OV. Pregnancy and umbilical cord pathology: structural and functional parameters of the umbilical cord. J Med Life. 2023 Aug;16(8):1282-1291. doi: 10.25122/jml-2023-0025.
Ilie C, Enătescu I, Ilie R, Căpitan F, Enătescu V, Margan M, et al. Patologia cordonului ombilical, cauză de hipoxie perinatală [Umbilical cord pathology caused by perinatal hypoxia]. Conexiuni Medicale [Medical Connections]. 2010;5(1):30-35. Romanian.
Li Z, Zhang S, Wang X, et al. Doppler ultrasound findings and clinical implications in pregnancies with umbilical cord abnormalities. J Matern Fetal Neonatal Med. 2022;35(6):968-974. doi: 10.1080/14767058.2021.1872023.
Zhang Y, Liu X, Li X, et al. The effects of umbilical cord abnormalities on placental function and fetal growth. Prenat Diagn. 2023;43(5):604-612. doi: 10.1002/pd.6211.
Lavric I, Bîtcă T, Bologan I. [Results of fetal vacuum extraction in modern practice]. In: [Biomedical and health research: quality, excellence and performance : Annual scientific conference of Nicolae Testemitanu SUMPh; 2021 Oct 20-22; Chişinău. Republic of Moldova]. Chișinau: Medicina; 2021. p. 399. ISBN 978-9975-82-223-7. Romanian.

