Introduction
Myeloid sarcomas (MS), formerly known as chloromas or granulocytic sarcomas, are extramedullary manifestations of myeloid neoplasms including acute myeloid leukemia (AML) and to a lesser extent myelodysplastic syndromes (MDS), and myeloproliferative neoplasms (MPN) [1]. By the World Health Organization’s classification definition, MS’s are composed of myeloid blasts with or without maturation and must present as a tumor mass that infiltrates and effaces the architecture of the extramedullary tissue [2]. MS can occur at any site, with different sites having different prognoses [3, 4]. The most commonly reported organs are lymph nodes, bones, soft tissues, and central nervous system [5, 6]. Atypical sites such as eyes, gall bladder, testis, and gastrointestinal system have also been reported [3, 7-11]. The majority of MS’s typically express myeloid (CD13, CD33, CD34, CD117, PU.1, Myeloperoxidase) or monocytic markers (CD11B, CD14, CD13, CD33, CD64, CD68, CD163, PU.1, Lysozyme) and lack expression of B/T cell markers (CD3, CD5, CD7, CD20, Pax-5) [2, 12]. Here we report an unusual biphasic MS relapse after Allo-SCT with an extensive “intravascular” component previously unreported in literature.
Case Report
A 59-year-old male presented to our institution with abdominal and bilateral lower limb pain in addition to systemic symptoms including fatigue, decreased appetite, and weight loss. The patient had a history of JAK2-V617F positive myelodysplastic syndrome/myeloproliferative neoplasm (MDS/MPN) and underwent allogeneic hematopoietic stem cell transplantation three years before his presentation. Physical examination showed multiple inguinal and bilateral lower limb skin lesions.
Material and methods
Complete blood count and peripheral blood smear were unremarkable except for normocytic anemia. A computerized tomography scan showed partial small and large bowel obstruction due to multiple colonic masses involving the transverse and descending colon, in addition to enlarged mesenteric and inguinal lymph nodes. Laparotomy revealed multiple masses involving all parts of the colon invading into the adjacent organs. Biopsy of the largest mass (7 cm) in the transverse colon was performed. Due to extensive disease, no tumor debulking was done.
Results
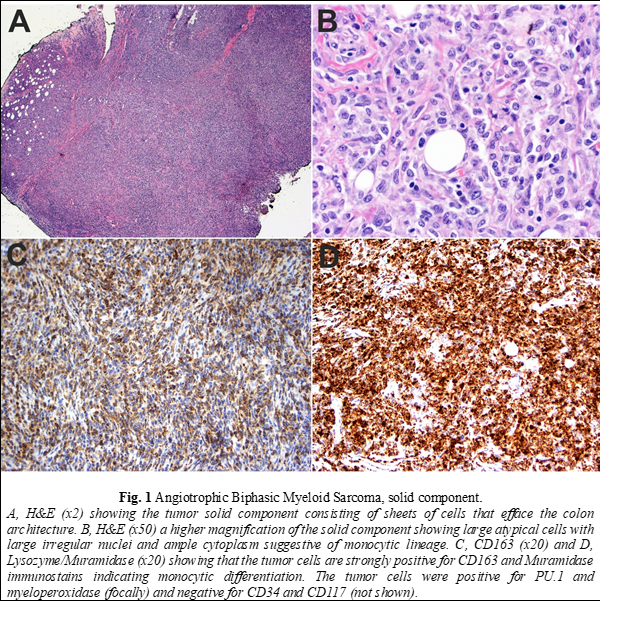
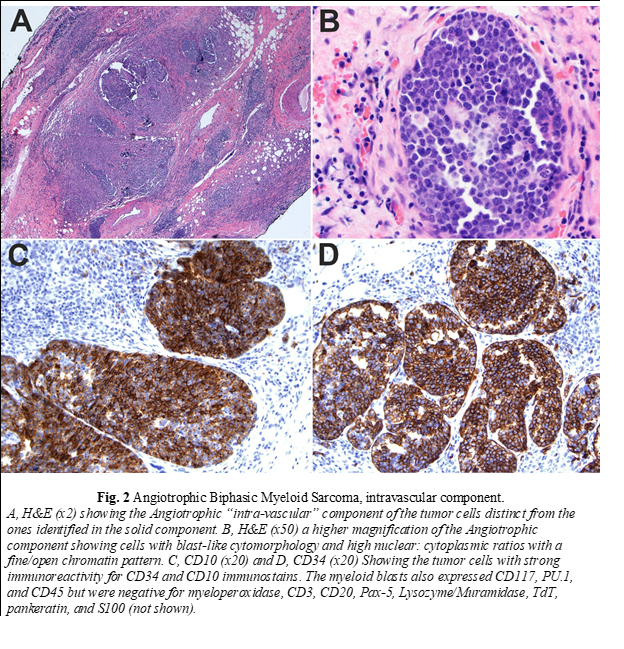
Histopathologic examination of the biopsy revealed a neoplasm consisting of both solid and intravascular components which were immunophenotypically and morphologically different (Fig. 1 and 2). The solid component (Fig. 1) was mainly composed of large cells of monocytic lineage which strongly expressed Lysozyme, CD163, CD45, CD68, and PU.1. Myeloid blasts (CD34, CD117) were only focally increased and comprised 3-5% of total cells. In contrast, the “intravascular” component (Fig. 2) was mainly composed of myeloid blasts with high nuclear: cytoplasmic ratios (NC) which strongly expressed CD10, CD34, CD45, partial/heterogeneous CD117, and PU.1, but were negative for CD3, CD20, Pax-5, Lysozyme, myeloperoxidase, and TdT. Next-generation sequencing (NGS) of DNA from the tissue biopsy revealed JAK2 (V617F) and KRAS (G12C) mutations. Fluorescence in situ hybridization (FISH) for MLL [11q23], PML/RARA [15q22/17q21.1], CBFB [16q22], and RUNX1/RUNX1T1 [21q22/8q22] exhibited normal signal patterns. Fine needle aspiration of an inguinal lymph node revealed a population of myeloid blasts that expressed CD45, CD13, CD33, CD34, CD38, CD71 (dim), CD117, and HLA-DR, and lacked expression of CD14 and CD64. Bone marrow aspiration and biopsy revealed markedly hypocellular marrow with decreased trilineage hematopoiesis and no increase in blasts. The patient was treated with FLAG (fludarabine, cytarabine, and filgrastim) followed by cytarabine and daunorubicin chemotherapy protocols. Despite the therapy, there was disease progression. Therefore, he was referred to hospice and died two months after surgery.
Discussion
MS occurs at an incidence of 2–9% in newly diagnosed AML patients [13, 14]. The prognosis of AML with concurrent MS carries an overall poor prognosis with a 5-year survival rate ranging between 20% and 30% [3]. It can be present upon the initial diagnosis of leukemia, or as a manifestation of relapsed disease after chemotherapy or hematopoietic cell transplantation [15, 16]. MS can be the first manifestation of disease relapse before relapse occurs within the marrow space, as seen in our case. In addition, isolated extramedullary relapse of AML more commonly occurs post allogeneic hematopoietic stem cell transplantation than post chemotherapy [16, 17]. The absence of concurrent leukemia makes the diagnosis of isolated MS a challenging one. MS’s are frequently misdiagnosed as large cell lymphomas with a high rate of misdiagnosis that can be up to 75% of cases [18].
Similar to AML, molecular events can also be identified in MS. Up to 55% of MS may contain cytogenetic abnormalities with the most common being trisomy 8 and inv(16) [19]. Other events including mutations (such as the common NPM1 mutation), gene rearrangements (such as KMT2A), and copy number variations (such as CBFB gene amplification) have been also reported. The mutation pattern in our case is unique. The finding of the JAK2 V617F mutation in the MS was consistent with the primary malignancy (MDS/MPN) and is proof of the molecular linkage between the two tumors. Although mutations in the KRAS genes are mostly seen in solid malignancies, it can be found in about 4-6% of AML cases [20]. In our case, the presence of those two driver mutations can represent the aggressive clinical behavior that results from acquiring additional driver mutations with progressive neoplastic clonal evolution.
The compound and complex nature of our case is also unique. The two components are distinct spatially, morphologically, and immunophenotypically. Spatially, each of the two components is localized to a particular compartment (solid tissue vs. vascular spaces). Morphologically, the intravascular component shows typical blast cytomorphology (fine/open chromatin with high N/C ratios) in contrast to the solid component, which shows a more differentiated cytomorphology (irregular nuclei with coarse chromatin and a relatively low N/C ratio). Immunophenotypically, the intravascular component expresses myeloid immature/blast markers (CD34 and CD117) with aberrant CD10 expression in contrast to the solid component, which lack those markers and instead express markers of monocytic lineage differentiation (Lysozyme/Muramidase, CD68, and CD163). Aberrant CD10 expression and other lymphoid-associated antigens have been reported to occur in AML in multiple studies [21-23]. Expression of aberrant lymphoid-associated antigens by AML has not been shown to alter the biologic behavior or response to therapy [24]. CD10 expression has been reported to occur with intravascular large B-cell lymphoma (ILBCL) and is usually associated with MUM-1 and BCL-6 expression, reflecting the germinal center origin of some cases [25-28]. In one study on 96 cases, CD10 expression was found in 13% of ILBCL cases [29].
ILBCL is an example of the isolated intravascular growth pattern of tumor cells [30-36]. In this phenomenon, there is a selective intraluminal proliferation of tumor cells in the small to medium-sized vessels due to a possible defect in adhesion molecules and homing receptor of malignant cells. Defects in molecules such as Intercellular Adhesion Molecule-1 and b1 integrin on the tumoral cells can impair the vascular transmigration of extracellular matrix required for tumor mass formation [37]. However, this phenomenon has not been reported to occur with MS. Although this report is limited for being a single case report, to the best of our knowledge, this is the first report in English literature of a compound biphasic myeloid sarcoma with an extensive “intravascular” component that is morphologically and immunophenotypically distinct.
Conclusion
In conclusion, this case report sheds light on the remarkable complexity and diversity of myeloid sarcomas (MS), an area of study that continues to present unique challenges and intriguing phenomena. The presentation of a biphasic MS, featuring both solid and "intravascular" components with distinct characteristics, adds a significant layer of novelty to the existing scientific literature on myeloid neoplasms. This case underscores the ongoing need for in-depth research to better comprehend the biology and behavior of myeloid sarcomas, especially in cases with atypical features and presentations. As we unravel the intricacies of these rare extramedullary manifestations, we move closer to a more comprehensive understanding of their clinical significance and, potentially, improved approaches to diagnosis and treatment.
Competing interests
None declared.
Authors’ contribution
SS devised the main conceptual ideas of the project. IA drafted the manuscript. All authors reviewed the manuscript and approved the final version.
Informed consent for publication
Written informed consent was not obtained from the patient for publication of this case report and any accompanying images, as it is not required by the regulations of our institution.
Funding
None.
Authors’ ORCID IDs
Ibrahim Abukhiran – https://orcid.org/0000-0003-0948-8391
Nancy Rosenthal – https://orcid.org/0000-0001-8120-9049
Sergei I. Syrbu – https://orcid.org/0000-0003-4620-7923
References
Solh M, Solomon S, Morris L, Holland K, Bashey A. Extramedullary acute myelogenous leukemia. Blood Rev. 2016;30(5):333-339. doi: 10.1016/j.blre.2016.04.001.
Swerdlow SH, Harris NL, et al., editors. WHO Classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: International Agency for Research on Cancer; 2017. 585 p.
Movassaghian M, Brunner AM, Blonquist TM, Sadrzadeh H, Bhatia A, Perry AM, Attar EC, Amrein PC, Ballen KK, Neuberg DS, Fathi AT. Presentation and outcomes among patients with isolated myeloid sarcoma: a surveillance, epidemiology, and end results database analysis. Leuk Lymphoma. 2015;56(6):1698-1703. doi: 10.3109/10428194.2014.963080.
Lan TY, Lin DT, Tien HF, Yang RS, Chen CY, Wu K. Prognostic factors of treatment outcomes in patients with granulocytic sarcoma. Acta Haematol. 2009;122(4):238-246. doi:10.1159/000253592.
Paydas S, Zorludemir S, Ergin M. Granulocytic sarcoma: 32 cases and review of the literature. Leuk Lymphoma. 2006;;47(12):2527-2541. doi: 10.1080/10428190600967196.
Bakst RL, Tallman MS, Douer D, Yahalom J. How I treat extramedullary acute myeloid leukemia. Blood. 2011;118(14):3785-3793. doi: 10.1182/blood-2011-04-347229.
Benjazia E, Khalifa M, Benabdelkader A, Laatiri A, Braham A, Letaief A, Bahri F. Granulocytic sarcoma of the rectum: report of one case that presented with rectal bleeding. World J Gastrointest Pathophysiol. 2010;1(4):144-146. doi: 10.4291/wjgp.v1.i4.144.
Narayan P, Murthy V, Su M, Woel R, Grossman IR, Chamberlain RS. Primary myeloid sarcoma masquerading as an obstructing duodenal carcinoma. Case Rep Hematol. 2012;2012:490438. doi: 10.1155/2012/490438.
Damodar S, Prashantha B, Gangoli A, Gopalakrishnan G, Jayanthi KJ. Granulocytic sarcoma of colon in a patient with acute promyelocytic leukemia. Indian J Hematol Blood Transfus. 2013;29(3):152-154. doi: 10.1007/s12288-012-0152-0.
Gupta S, Chawla I, Singh V, Singh K. Granulocytic sarcoma (chloroma) presenting as colo-colic intussusception in a 16-year-old boy: an unusual presentation. BMJ Case Rep. 2014:2014:bcr2014206138. doi: 10.1136/bcr-2014-206138.
Rogers R, Ettel M, Cho M, Chan A, Wu XJ, Neto AG. Myeloid sarcoma presenting as a colon polyp and harbinger of chronic myelogenous leukemia. World J Gastrointest Oncol. 2016;8(3):321-325. doi: 10.4251/wjgo.v8.i3.321.
Seifert RP, Bulkeley W 3rd, Zhang L, Menes M, Bui MM. A practical approach to diagnose soft tissue myeloid sarcoma preceding or coinciding with acute myeloid leukemia. Ann Diagn Pathol. 2014;18(4):253-260. doi: 10.1016/j.anndiagpath.2014.06.001.
Wiernik PH, Serpick AA. Granulocytic sarcoma (chloroma). Blood. 1970;35(3):361-369.
Liu PI, Ishimaru T, McGregor DH, Okada H, Steer A. Autopsy study of granulocytic sarcoma (chloroma) in patients with myelogenous leukemia, Hiroshima-Nagasaki 1949-1969. Cancer. 1973;31(4):948-955. doi: 10.1002/1097-0142(197304)31:4<948::aid-cncr2820310428>3.0.co;2-n.
Neiman RS, Barcos M, Berard C, Bonner H, Mann R, Rydell RE, Bennett JM. Granulocytic sarcoma: a clinicopathologic study of 61 biopsied cases. Cancer. 1981;48(6):1426-1437. doi: 10.1002/1097-0142(19810915)48:6<1426::aid-cncr2820480626>3.0.co;2-g.
Solh M, DeFor TE, Weisdorf DJ, Kaufman DS. Extramedullary relapse of acute myelogenous leukemia after allogeneic hematopoietic stem cell transplantation: better prognosis than systemic relapse. Biol Blood Marrow Transplant. 2012;18(1):106-112. doi: 10.1016/j.bbmt.2011.05.023.
Shimizu H, Saitoh T, Hatsumi N, Takada S, Handa H, Jimbo T, Sakura T, Miyawaki S, Nojima Y. Prevalence of extramedullary relapses is higher after allogeneic stem cell transplantation than after chemotherapy in adult patients with acute myeloid leukemia. Leuk Res. 2013;37(11):1477-1481. doi: 10.1016/j.leukres.2013.08.017.
Meis JM, Butler JJ, Osborne BM, Manning JT. Granulocytic sarcoma in nonleukemic patients. Cancer. 1986;58(12):2697-2709. doi: 10.1002/1097-0142(19861215)58:12<2697::aid-cncr2820581225>3.0.co;2-r
Alexiev BA, Wang W, Ning Y, Chumsri S, Gojo I, Rodgers WH, Stass SA, Zhao XF. Myeloid sarcomas: a histologic, immunohistochemical, and cytogenetic study. Diagn Pathol. 2007;2(1):42. doi: 10.1186/1746-1596-2-42.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. doi: 10.1126/scisignal.2004088.
Bavikatty NR, Ross CW, Finn WG, Schnitzer B, Singleton TP. Anti-CD10 immunoperoxidase staining of paraffin-embedded acute leukemias: comparison with flow cytometric immunophenotyping. Human Pathol. 2000;31(9):1051-1054. doi: 10.1053/hupa.2000.6276.
Weinberg OK, Hasserjian RP, Li B, Pozdnyakova O. Assessment of myeloid and monocytic dysplasia by flow cytometry in de novo AML helps define an AML with myelodysplasia-related changes category. J Clin Pathol. 2017;70(2):109-115. doi: 10.1136/jclinpath-2016-203863.
Shahni A, Saud M, Siddiqui S, Mukry SN. Expression of aberrant antigens in hematological malignancies: a single center experience. Pakistan J Med Sci. 2018;34(2):457-462. doi: 10.12669/pjms.342.13996.
Drexler HG, Thiel E, Ludwig WD. Acute myeloid leukemias expressing lymphoid-associated antigens: diagnostic incidence and prognostic significance. Leukemia. 1993;7(4):489-498.
Estalilla OC, Koo CH, Brynes RK, Medeiros LJ. Intravascular large B-cell lymphoma. A report of five cases initially diagnosed by bone marrow biopsy. Am J Clin Pathol. 1999;112(2):248-255. doi: 10.1093/ajcp/112.2.248.
Sakakibara A, Inagaki Y, Imaoka E, Ishikawa E, Shimada S, Shimada K, Suzuki Y, Nakamura S, Satou A, Kohno K. Autopsy case report of intravascular large B-cell lymphoma with neoplastic PD-L1 expression. J Clin Exp Hematopath. 2018;58(1):32-35. doi: 10.3960/jslrt.17037.
Li Q, Li J, Yang K, Peng Y, Xiang Y, Sun S, Zeng J, Zhang X, Wang J. EBV-positive intravascular large B-cell lymphoma of the liver: a case report and literature review. Diagn Pathol. 2020;15(1):72. doi: 10.1186/s13000-020-00989-x.
Masood S, Vijayan K, Wheeler YY. Primary lung intravascular large B-Cell lymphoma clinically mimicking sarcoidosis: a rare case report and review of literature. Respir Med Case Rep. 2020;29:100989. doi: 10.1016/j.rmcr.2019.100989.
Murase T, Yamaguchi M, Suzuki R, Okamoto M, Sato Y, Tamaru J, Kojima M, Miura I, Mori N, Yoshino T, Nakamura S. Intravascular large B-cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood. 2007;109(2):478-485. doi: 10.1182/blood-2006-01-021253.
Ameli F, Nili Ahmad Abadi F, Saffar H. Intravascular large B-cell lymphoma: a report of two cases. Iran J Pathol. 2020;15(4):346-350. doi: 10.30699/ijp.2020.119590.2299.
Cenk H, Sarac G, Karadağ N, Berktas HB, Sahin I, Sener S, Kisaciik D, Kapicioglu Y. Intravascular lymphoma presenting with paraneoplastic syndrome. Dermatol Online J. 2020;26(8):13030/qt08252906.
Enzan N, Kitadate A, Suzuki H, Yoshioka M, Narita K, Tanaka A, Matsue K. Intravascular large B-cell lymphoma diagnosed by incisional random skin biopsy after failure of diagnosis using punch biopsy: a case report. J Cutan Pathol. 2021;48(4):589-591. doi: 10.1111/cup.13877.
Kurek C, Ehsman S, Brighton T, Young K. Incidental intravascular large B-cell lymphoma in a renal cell carcinoma. Pathology. 2020;52(6):731-734. doi: 10.1016/j.pathol.2020.06.012.
Minish JM, Kelkar AH, Mehta AR, Gaffar M, Dang NH. Intravascular large B-Cell lymphoma presenting as acute axonal polyneuropathy: a case report and literature review. J Investig Med High Impact Case Rep. 2020;8:2324709620959997. doi: 10.1177/2324709620959997.
Rodriguez-Justo M, Attygalle AD, Munson P, Roncador G, Marafioti T, Piris MA. Angioimmunoblastic T-cell lymphoma with hyperplastic germinal centres: a neoplasia with origin in the outer zone of the germinal centre? Clinicopathological and immunohistochemical study of 10 cases with follicular T-cell markers. Mod Pathol. 2009;22(6):753-761. doi: 10.1038/modpathol.2009.12.
Yu H, Chen G, Zhang R, Jin X. Primary intravascular large B-cell lymphoma of lung: a report of one case and review. Diagn Pathol. 2012;7:70. doi: 10.1186/1746-1596-7-70.
Kinoshita M, Izumoto S, Hashimoto N, Kishima H, Kagawa N, Hashiba T, Chiba Y, Yoshimine T. Immunohistochemical analysis of adhesion molecules and matrix metalloproteinases in malignant CNS lymphomas: a study comparing primary CNS malignant and CNS intravascular lymphomas. Brain Tumor Pathol. 2008;25 (2):73-78. doi: 10.1007/s10014-008-0232-x.