Introduction
The use of medicinal plants has a long history, dating back to ancient times, with its practice widespread in different regions around the world. Both the past and the present are marked by the fundamental importance of the use of plants in ethnomedicine [1]. Medicinal plants are used to treat a wide range of disorders, diseases and illnesses: dermal, cardiovascular, endocrine, gastrointestinal, genito-urinary, respiratory, musculoskeletal, hepatic, including cancer [2]. The therapeutic effects of medicinal plants are often attributed to secondary metabolites, but it should be noted that carotenoid and chlorophyll pigments also play a significant role in the prophylaxis and treatment of various diseases [1].
Carotenoids and chlorophylls are the most common pigments in nature, providing a wide range of colors (yellow, orange, red, purple, green) to plants. Carotenoids are tetraterpene pigments, predominantly composed of 8 isoprene units with a backbone of 40 carbon atoms. Carotenoids are divided into 2 groups: carotenes and xanthophylls. Carotenes, such as α, β-carotene, ψ-carotene (γ-carotene) and lycopene, are represented by hydrocarbons. About 50 types of carotenes are present in nature [3]. On the other hand, xanthophylls, such as β-cryptoxanthin, lutein, zeaxanthin, astaxanthin, fucoxanthin and peridinin, are carotenoids, which contain oxygen atoms in the form of hydroxy, carbonyl, aldehyde, carboxyl, epoxide and furanoxide groups in its molecules, which also confer pronounced antioxidant properties [4]. As of 2018, more than 800 types of xanthophylls have been reported in nature, some of which are present as fatty acid esters, glycosides, sulfates and protein complexes [3, 4].
Chlorophylls have a porphyrin ring at their base, to which a long carbon chain (phytol) is attached, and a magnesium ion in the center. The purpose of the porphyrin ring is to capture solar energy and the magnesium ion acts as an electron acceptor. There are several types of chlorophyll, such as chlorophyll a, b, c, d and chlorophyll e [1], with different colour shades, such as for chlorophyll a – bluish green, and chlorophyll b – yellowish green.
In the plant body, carotenoids and chlorophylls are essential compounds in providing two major physiological processes such as photosynthesis (being part of the light-harvesting complex) and protection against photo-oxidative damage in cells [4, 5].
Carotenoids and chlorophylls capture light energy in the blue-red spectral range (400-650 nm) and excite electrons in pigment molecules. After absorbing light energy, the excited electrons in chlorophyll are used to carry out the two phases of photosynthesis: light and dark. The first phase of photosynthesis is also known as light-dependent reactions, which take place in the thylakoid membrane of chloroplasts and is responsible for the production of ATP and NADPH. The second phase of photosynthesis is known as light-independent reactions, also called the Calvin cycle, which takes place in the stroma of chloroplasts. In this phase, ATP and NADPH produced in the light-dependent reactions are used to achieve the production of glucose and oxygen [1].
An important function of carotenoids is the absorption of excess energy from chlorophylls by triplet-triplet transfer and the release of excess energy by polyene vibration. Triplet-triplet transfer is an essential higher energy state in photoprotection. Reactive types of oxygen, such as singlet oxygen, hydroxyl radicals and superoxide anion radicals, are produced by oxygen and light during photosynthesis. Carotenoids with more than 11 conjugated double bonds show a marked ability to quench singlet oxygen [6].
Another major function of carotenoids is the synthesis of hormones (growth regulators), which are formed by their oxidative cleavage to form apocarotenoids. Apocarotenoids perform such vital functions as growth regulators, stress factor signalers and fungal attack agents. One of the best known phytohormones is abscisic acid, which regulates plant growth, seed dormancy, embryo maturation and germination, cell division and elongation, stomatal cell movement, floral growth and responses to biotic and abiotic stresses [3, 5]. Carotenoids are also present in non-photosynthesizing plant organs such as roots, flowers, fruit pericarp and seeds, acting as photoprotectants, antioxidants, color attractants and plant hormone precursors [7].
Approximately 50 types of carotenoids are found in the typical human diet, of which about 20 can be detected in blood (plasma or serum) following ingestion [8]. Carotenoids can be found in several human organs such as liver, adrenal glands, ovaries, skin, lungs, testes, prostate and blood serum. The distribution of carotenoids in human organs shows specificity. Lutein and zeaxanthin are found on the surface of skin and subcutaneous tissue in an esterified form and act as UV absorbers and singlet oxygen quenchers [9]. Xanthophylls, such as β-cryptoxanthin, lutein and zeaxanthin, are found in the brain [10]. In the eye, lutein and zeaxanthin are present as macular pigments [7]. Lycopene accumulates in the prostate [6].
The first report on the efficacy of dietary β-carotene in reducing human cancer rates [6] was followed by multiple preclinical and clinical studies demonstrating the role of intake of carotenoid-containing leafy green vegetables and fruits in reducing cancer risk. For example, the fruit of mandarin Citrus unshiu, cultivar ‘Satuma’ is characterized with high content of β-cryptoxanthin, and may be associated with reduced cancer risk. Consumption of lycopene has shown to improve health outcomes in prostate cancer patients. In addition, clinical studies have shown, that administration of natural carotenoid complex (mixture of α and β-carotene, lutein and lycopene) and α-tocopherol significantly suppresses hepatoma development in patients with hepatitis virus-induced cirrhosis. Carotenoids have also been reported to contribute to the prevention of cardiovascular disease, diabetes, obesity and several lifestyle-related diseases. In addition, carotenoids improve resistance, boost immunity and especially skin quality [4].
The bioactivity of chlorophylls is attributed to their ability to act as antioxidants, antimutagens and anticarcinogens. The unique chemical structure allows chlorophylls to scavenge harmful free radicals, mitigate DNA damage and modulate cellular processes involved in the development of disease. In addition, their hydrophobic side chains facilitate interactions with biological membranes, influencing cellular uptake and signalling pathways [1].
Species of the g. Galium, with a long history as medicinal plants in traditional medicine, have attracted the attention of researchers, who have scientifically demonstrated that their carotenoids and chlorophylls content provides biologically active substances with antioxidant, antiradiant and energizing properties [11-13]. Given these findings, our study aims to determine the content of carotenoid and chlorophyll pigments in different organs of G. verum and G. aparine from the wild flora of the Republic of Moldova.
Material and methods
Plant material. Different vegetal products (stems, leaves, flowers and aerial parts), collected during the flowering period from 2 species of G. verum and G. aparine from the spontaneous flora of the Republic of Moldova, were used for the study of pigments. The species were collected from the North of the Republic of Moldova: G. verum were collected from Brinzeni village, Edinet district (48°5′0″ North 27°10′28″ East), and G. aparine – from Taul village, Donduseni district (48°12′57″ North 27°40′22″ East). The collected material was dried and conditioned according to the technical normative requirements [14].
Preparation of plant extracts. The dried plant product was shredded using a grinder and the powder was sieved through a 0.5 mm pore sieve. Extracts were obtained by repeated extraction of the pulverized plant products with a mixture of ethanol:water at two concentrations (60 and 95%, w/w) for half an hour at each extraction step until the plant products were exhausted. Extraction was performed with a water bath coupled with a condenser. Extractive solutions obtained were concentrated at 40°C using a rotary evaporator – Laborota 4011.
Chemical and reagents. Ethyl alcohol was used as the solvent to extract the carotenoids and chlorophylls at two concentrations: 60 and 95%.
Sphectrophotometric assay. The optical density of the extracts obtained was measured by Metertech UV/VIS SP 8001 spectrophotometer at different wavelengths.
For the determination of carotenoids, the optical density of the extract was determined at 448 nm, after which the absorbance was substituted in formula number 1 [15].
Formula for the determination of carotenoids:
(1) ![]()
where: A – absorbance; m – raw material weight; w – yield of plant product on drying (G. verum – 10.15; G. aparine – 11.12).
Chlorophyll optical density was determined at 664 nm for chlorophyll a, and at 649 nm for chlorophyll b. To determine the chlorophyll content, absorbance was substituted in formulas 2 and 3 for chlorophyll a and b, respectively.
Chl a = 13.36×A664−5.19×A649 (2),
Chl b = 27.43×A649−8.12×A664 (3).
Statistical analysis. All determinations were made in triplicate, and the results are reported as mean values ± standard deviation (SD) using Microsoft Excel 2021.
Results
The carotenoid contents of the analyzed alcohol extracts, calculated using formula 1, are presented numerically in Table 1 and diagrammatically in Figures 1 and 2.
Table 1. Carotenoid content (±SD) in different organs of G. verum and G. aparine species | ||||||||
Species, organ/ Analyzed extract
| Carotenoid content (mg/%) | |||||||
Organs of G. verum | Organs of G. aparine | |||||||
stems | leaves | flowers | aerial parts | stems | leaves | flowers | aerial parts | |
Alcoholic extract (60%) | 13.39 ± 0.07 | 20.03 ± 0.11 | 20.81 ± 0.1 | 38.03 ± 0.13 | 6.23 ± 0.09 | 15.91 ± 0.06 | 18.36 ± 0.07 | 20.92 ± 0.09 |
Alcoholic extract (95%) | 23.85 ± 0.08 | 33.98 ± 0.11 | 35.1 ± 0.07 | 52.66 ± 0.09 | 7.23 ± 0.07 | 20.03 ± 0.06 | 24.26 ± 0.04 | 27.38 ± 0.11 |
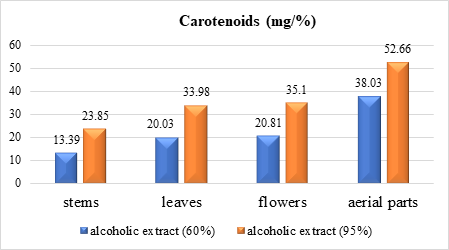 |
Fig. 1 Carotenoid content extracted with 60% and 95% ethyl alcohol from different organs of G. verum species |
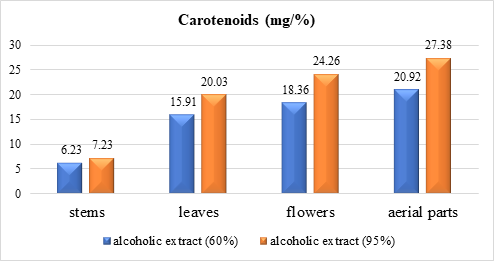 |
Fig. 2 Carotenoid content extracted with 60% and 95% ethyl alcohol from different organs of G. aparine species |
Analysis of the obtained data (Table 1 and Fig. 1, 2) shows that 95% ethyl alcohol facilitates better extraction than 60% ethyl alcohol for all organs in both species of genus Galium. When comparing the results, we established that in extracts of organs of G. verum species, the content of carotenoids is obviously higher than in G. aparine (stems – 23.85 and 7.23; leaves – 33.98 and 20.03; flowers – 35.1 and 24.26; aerial parts – 52. 66 and 27.38).
The data in Table 1, Figure 1 and 2 indicate that the carotenoid content (mg/%) with 95% alcohol ranges from 23.85 for stems to 52.66 in aerial parts for G. verum and from 7.23 to 27.38 for G. aparine, respectively. Note that carotenoid content correlates with plant organ in the same consecutiveness for both species (decreasing): the highest values are for aerial parts, followed by flowers, leaves and the lowest are recorded for stems.
The contents of chlorophylls a and b from the alcoholic extracts from vegetal products of G. verum and G. aparine, calculated according to formulas 2 and 3, expressed in mg/l, are given in Table 2.
Table 2. Content (±SD) of chlorophyll a and b pigments in the organs of G. verum and G. aparine species | ||||||||
Species | Chlorophyll content (mg/l) | |||||||
Alcoholic extract 60% | Alcoholic extract 95% | |||||||
G. verum | stems | leaves | flowers | aerial parts | stems | leaves | flowers | aerial parts |
Chl a | 0.13 ± 0.09 | 0.23 ± 0.13 | 0.26 ± 0.18 | 0.33 ± 0.1 | 0.22 ± 0.12 | 1.09 ± 0.15 | 0.5 ± 0.05 | 1.2 ± 0.08 |
Chl b | 0.26 ± 0.16 | 0.51 ± 0.15 | 0.65 ± 0.12 | 0.67 ± 0.08 | 0.23 ± 0.18 | 1.64 ± 0.16 | 0.74 ± 0.07 | 1.9 ± 0.05 |
| Alcoholic extract 60% | Alcoholic extract 95% | ||||||
G. aparine | stems | leaves | flowers | aerial parts | stems | leaves | flowers | aerial parts |
Chl a | 0.34 ± 0.02 | 0.41 ± 0.03 | 0.66 ± 0.016 | 1.1 ± 0.05 | 1.07 ± 0.04 | 2.28 ± 0.08 | 1.85 ± 0.11 | 2.85 ± 0.19 |
Chl b | 0.48 ± 0.1 | 0.58 ± 0.18 | 0.89 ± 0.02 | 1.57 ± 0.13 | 1.46 ± 0.22 | 2.3 ± 0.19 | 1.87 ± 0.05 | 4.33 ± 0.14 |
Note: Chl a – Chlorophyll a, Chl b – Chlorophyll b; SD – standard deviation | ||||||||
The analysis of the chlorophyll content in the organs of Galium species shows a higher content (mg/l) of chlorophyll a and b in the aerial parts of G. aparine compared to G. verum in the 95% alcoholic extract (respectively chlorophyll a: aerial parts – 2.85 and 1.2; leaves – 2.28 and 1.09; flowers – 1.85 and 0.5; stems – 1.07 and 0.22 and chlorophyll b: aerial parts – 4.33 and 1.9; leaves – 2.3 and 1.64; flowers – 1.87 and 0.74 and stems – 1.46 and 0.23).
Discussion
This is the first study that analyzes and compares the content of chlorophyll and carotenoid pigments in the organs (stems, leaves, flowers and aerial parts) of 2 species of the genus Galium (G. verum and G. aparine) from the wild flora of the Republic of Moldova.
Previous studies [15] came to the same conclusion, that 95% ethyl alcohol facilitates better extraction of chlorophyll and carotenoid pigments compared to 60% ethyl alcohol.
Comparing the obtained results, we establish that in the alcoholic extracts from different organs of G. verum, the carotenoid content is obviously higher than in G. aparine with quantitative prevalence in aerial parts (aerial parts – 52.66 and 27.38; flowers – 35.1 and 24.26; leaves – 33.98 and 20.03; stems – 23.85 and 7.23). The comparative study of 6 species of genus Galium [12] similarly indicates higher carotenoid content in aerial parts of G. verum compared to G. aparine and 4 other studied species.
Carotenoids have a biologically determined role in energy transmission and singlet oxygen quenching chain, precursors of growth regulators [3, 4]. Additionally, numerous studies from different laboratories has been demonstrated the contribution of carotenoids in determining of antioxidant potential [7]. Recent studies identified the antimicrobial and antifungal activity of carotenoid-containing lipid complex in Galium species: G. aparine – the highest against strains of Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis and the lowest against Escherichia coli. G. verum exhibited strong sensitivity against B. subtililis and moderate activity against Proteus vulgaris [12]. The antifungal action denotes that the extract with carotenoids and chlorophylls from G. aparine has the highest sensitivity to Candida albicans, while G. verum was characterized with no significant antifungal effect.
Based on a comparison of the obtained results, we establish that the alcoholic extracts from the organs of G. aparine species are richer in chlorophylls a and b than those of G. verum, and the amount of chlorophyll b is higher than chlorophyll a, which has been mentioned in other studies [11].
Chlorophyll a is the primary bluish green pigment required for photosynthesis (it absorbs light with wavelength 430-662 nm from the orange-red and blue-violet regions of the electromagnetic spectrum, thus transferring energy to the reaction center and donating 2 excited electrons to the electron transport chain), and chlorophyll b – a yellowish green, accessory pigment, which is not always present in photosynthesizing organisms, but absorbs blue light (with peak absorption at around 453 nm) [1] and thus contributes to broadening the absorption spectrum of organisms. In this way, organisms can absorb more energy from the high-frequency blue light part of the spectrum [16]. The presence of chlorophyll b in cells demonstrates the adaptation of organisms to light-deficient conditions, helping them to convert a wider range of solar energy into the chemical one [17]. Thus, the higher values of chlorophyll b relative to a in all extracts analyzed in Galium sp., denote their high potential adaptability to light conditions to ensure an efficient photosynthetic process.
Besides the biological role of chlorophylls in the process of photosynthesis and the starting link for the biosynthesis of different chemical compounds [17], the therapeutic role of chlorophylls has been demonstrated through various studies, based on their ability to act as antioxidants, antimutagens and anticarcinogens, due to their unique chemical structure, which allows chlorophylls to scavenge harmful free radicals, attenuate DNA damage and modulate cellular processes involved in the development of diseases [1]. The antioxidant properties of G. verum were studied, on aerial parts extract, by 3 methods (DPPH, determination of OH radical scavenging activity and determination of hydrogen peroxide scavenging activity), the result of which shows that the extract with higher chlorophyll content has higher antioxidant capacity [11].
The chlorophyll a and b complex of G. aparine is characterized with half microbial inhibitory and bactericidal properties against E. coli strain, compared to Chlorophyllipt (the drug is a mixture of chlorophylls derived from eucalyptus leaves, which has bacteriostatic and bactericidal action, especially against staphylococci, including antibiotic-resistant strains, and has anti-inflammatory properties) but with low antifungal activity against C. albicans compared to Chlorophyllipt [12].
Conclusions
Spectrophotometric determination of carotenoids (mg/%) in different vegetal products of G. verum and G. aparine species shows that the highest content of carotenoids is extracted with 95% ethyl alcohol in G. verum: aerial parts (52.66), flowers (35.1), leaves (33.98), stems (23.85), followed by carotenoids of G. aparine.
Comparative analysis of chlorophyll a and b content (mg/l) in organs of the species presents their maximum concentration in aerial parts of both species (G. verum – chlorophyll a – 1.9 and chlorophyll b – 1.2 and G. aparine – 4.33 and 2.85, respectively) than in flowers, leaves and stems. In all analyzed extracts the content of chlorophyll b prevails numerically over chlorophyll a.
Competing interests
None declared.
Authors’ contributions
AO collected the data, performed the experimental part; MCT coordinated experimental activity and approved the manuscript; TC interpreted the data and drafted the manuscript; All authors revised and approved the final version of the manuscript.
Acknowledgements and funding
The study was carried out with funding from the project „Development of new pharmaceutical products from local raw material” (no. 080301).
Provenance and peer review
Not commissioned, externally peer review.
Authors’ ORCID IDs
Angelica Ohindovschi – https://orcid.org/0000-0001-5132-0782
Tatiana Calalb – https://orcid.org/0000-0002-8303-3670
Maria Cojocaru-Toma – https://orcid.org/0000-0002-8255-9881
References
Martins T, Novo Barros A, Rosa E, Antunes L. Enhancing health benefits through chlorophylls and chlorophyll-rich agro-food: a comprehensive review. Molecules. 2023;28(14):5344. https://doi.org/10.3390/molecules28145344.
Ravichandran S, Manju Bhargavi K, Rai A, et al. Medicinal plants for curing human diseases. Chin Med. 2023:6(1):570. doi: 10.18282/i-cm.v6i1.570.
Britton G, Liaaen-Jensen S, Pfander H. Introduction and guidelines on the use of the Handbook. In: Britton G, Liaaen-Jensen S, Pfander H, editors. Carotenoids. Basel: Birkhäuser; 2004. p. 1-33. https://doi.org/10.1007/978-3-0348-7836-4_1.
Maoka T. Recent progress in structural studies of carotenoids in animals and plants. Arch Biochem Biophys. 2009;483(2):191-195. doi: 10.1016/j.abb.2008.10.019.
Swapnil P, Meena M, Singh K, et al. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr Plant Biol. 2021;26:100203. doi: 10.1016/j.cpb.2021.100203.
Maoka T. Carotenoids as natural functional pigments. J Nat Med. 2020;74(1):1-16. doi: 10.1007/s11418-019-01364-x.
Britton G, Liaaen-Jensen S, Pfander H. Carotenoids. Vol. 4: Natural functions. Basel: Birkhäuser; 2008. 370 p. ISBN 978-3-7643-7498-3.
Khachik F, Beecher G, Goli M, Lusby W, Smith J. Separation and identification of carotenoids and their oxidation products in the extracts of human plasma. Anal Chem. 1992;64(18):2111-2122. doi: 10.1021/ac00042a016.
Wingerath T, Sies H, Stahl W. Xanthophyll esters in human skin. Arch Biochem Biophys. 1998;355(2):271-274. doi: 10.1006/abbi.1998.0734.
Craft N, Haitema T, Garnett K, Fitch K, Dorey C. Carotenoid, tocopherol, and retinal concentration in elderly human brain. J Nutr Health Aging. 2004;8(3):156-162.
Lakic N, Mimica-Dukic N, Isak J, Božin B. Antioxidant properties of Galium verum L. (Rubiaceae) extracts. Cent Eur J Biol. 2010;5(3):331-337. https://doi.org/10.2478/s11535-010-0022-4.
Ilyina T, Goryachaya O, Toryanik E. Antimicrobial activity of the genus Galium L. Pharmacogn Commun. 2016:6(1):42-47. doi: 10.5530/pc.2016.1.8.
Turcov D, Barna S. Antioxidants from Galium verum as ingredients for the design of new dermatocosmetic products. Plants. 2022;11(19):2454. https://doi.org/10.3390/plants11192454.
Nistreanu A. Farmacognozie [Pharmacognosy]. Chișinău: Tipografia Centrală; 2000. 672 p. Romanian.
Trineeva OV, Slivkin AI. Validatsiia metodiki opredeleniia karotinoidov v plodakh oblepikhi razlichnykh sposobov konservatsii [Validation of the methodology for the determination of carotenoids in sea buckthorn fruits of different preservation methods]. Vestnik VGU. Seriia: Khimiia. Biologiia. Farmatsiia [Proceedings of Voronezh State University. Series: Chemistry. Biology. Pharmacy]. 2016;(2):145-151. Russian.
Voitsekhovskaja OV, Tyutereva EV. Chlorophyll b in angiosperms: functions in photosynthesis, signaling and ontogenetic regulation. J Plant Physiol. 2015;189:51-64. https://doi.org/10.1016/j.jplph.2015.09.013.
Lakna P. Difference between chlorophyll A and B, definition, characteristics, role, comparison [Internet]. In: Pedia.com. 2017 Apr 11 [cited 2024 Jul 12]. Available from: https://pediaa.com/difference-between-chlorophyll-a-and-b/
Murariu A, Stratu A, Zamfirescu O. Ecophysiological research on some medicinal plants from Bălteni forest (Vaslui county). In: 4th Conference on Medicinal and Aromatic Plants of South-East European Countries; 2006, Iasi, Romania: Book of abstracts. Iasi: Alma Mater; 2006. p. 24.

