Introduction
The clinical concept that argues that the activity of the lateral pterygoid muscle, being disturbed, plays an important role as an etiological factor in temporomandibular joint (TMJ) dysfunctions is still widely accepted. It also remains a decisive factor in the correct choice of the treatment plan. However, due to the fact that few rigorous studies and clear evidence have been conducted and presented to fully support this concept, it continues to be a very controversial one [1, 2]. Patients with temporomandibular disorders (TMD) complain of pain in the TMJ and/or masticatory muscles, limitations and sounds during mandibular movements. Temporomandibular disorders are a non-specific collective term used to describe a heterogeneous group of pathological conditions located in the territory of the stomatognathic/masticatory system. These are considered musculoskeletal disorders, which cause pain during the performance of the function (mastication, speech, swallowing), with increased sensitivity at the level of masticatory muscles and/or the TMJ, along with possible limitations of the range of motion and the occurrence of joint noises and otological symptoms [1, 3-6]. Current data demonstrate that TMD is one of the most commonly diagnosed forms of musculoskeletal pain in all age groups. Ethnicity, age, geographic location, and the time of assessment influence the level of prevalence, causes, factors, and spectrum of clinical manifestation of the pathology [7, 8]. In adolescents, the prevalence is a controversial subject, considering that adults are more frequently affected by TMD, but at the same time, a significantly increased incidence is noted in subjects with mixed dentition (worldwide incidence – 25%, and in the developed countries – between 2% and 6%) [1, 3-5, 9]. One theory claims that in TMD, the lateral pterygoid muscle becomes hyperactive, hypoactive, or that there is a miscoordination between the superior and inferior branches of the muscle, or that there is a disturbance during the execution of the role of the muscle to control and stabilize the TMJ [1, 10]. However, a rigorous review of the literature indicates that no clear scientific evidence is yet available to suggest that the function of the lateral pterygoid muscles is somehow disrupted in TMD. Moreover, the muscle's role during the execution of its normal function has also been questioned and remains a matter of controversy.
Materials and methods
For this literature review, scientific articles published between 2000 and 2023 were considered and studied using the following electronic databases: PubMed, MEDLINE, Google Scholar, BIR Publications, ScienceDirect. Keywords such as „lateral pterygoid muscle”, “temporomandibular joint”, and “TMJ dysfunction” were used. A total of 137 articles were studied, out of which 12 were duplicate articles, 45 articles presented studies performed on cadavers, 21 articles did not provide sufficient data, and 19 articles were conducted before the year 2000. The exclusion criteria included studies performed on cadavers, studies conducted before the year 2000, and studies that did not present trustworthy information on the topic under study.
Results and discussions
Anatomical variations of the lateral pterygoid muscle. In recent years, several studies have been carried out with the purpose of proving a theory that there may be a third fascicle of the lateral pterygoid muscle or that the two fascicles may be anatomically inserted differently at the level of the disc and condyle among different individuals. To study the anatomical variations of the lateral pterygoid muscle and its insertion variations, analyzes were performed using magnetic resonance imaging (MRI). In a 2013 study by Valenzuela et al., 698 patients underwent MRI analysis of the TMJ. Three types of muscle insertion of the superior fascicle of the muscle have been described (Table 1). The first type of muscle insertion involved muscle fibers inserting onto the articular disc. The second type of muscle insertion was at the level of the articular disc and condyle, and the third type was at the level between the articular disc and the articular capsule (Figure 1) [11, 12]. In another similar study conducted in 2016 by Eberhard et al., in which 382 patients with articular disc dislocation were evaluated by MRI, the results showed that the prevailing type of muscle insertion of the superior fascicle among subjects was type 2, at 67%, and the one with the lowest prevalence was type 1, at 7.6%. MRI images were taken in the position of maximum intercuspation (MI) and full opening of the oral cavity [13].
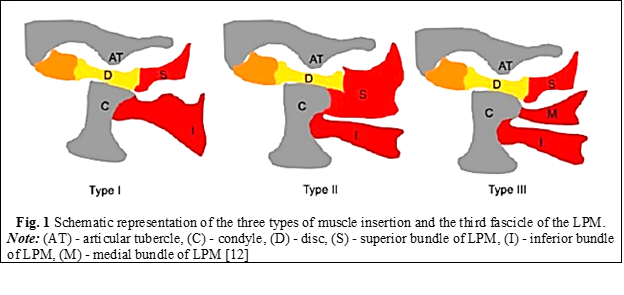
Table 1. Types of muscle insertion of the lateral pterygoid muscle. Litko classification [13] | ||
Type of insertion | Bundle | Insertion |
Type 1 | Superior Inferior | Disc Condyle |
Type 2* | Superior Inferior | Disc and condyle Condyle |
Type 3 | Superior Medial Inferior | Disc Condyle Condyle |
Note: (*) – the highest prevalence with 76% of subjects according to Eberhard et al. studies. | ||
Function of the lateral pterygoid muscles. The international theory about the anatomy of the lateral pterygoid muscle says that it is a masticatory muscle, which is part of the dento-maxillary system. It is considered to be an important masticatory component, in some sources even the main one, either structurally or functionally, due to its direct insertion at the level of the components of the temporomandibular joint. In some temporomandibular pathologies or dysfunctions, the muscle would be described as being directly or indirectly involved. Directly, it is described as the muscle that specifically causes TMD. The dysfunction involves the alteration of the movement of the joint and causes pain in neighboring structures, which are nervous, vascular or bone [11, 14, 15]. Pathologically, it is described that at the level of the joint there would be an impediment or an inconsistency between the elements, but the most affected component would be the articular disc, which is directly associated with the lateral pterygoid muscle that causes the anterior dislocation of the disc [11]. Mechanically, this joint dysfunction produces an anterior disc dislocation, which, in turn, compresses with the bony tissues, causing a bony stop for the articular condyle, reducing joint space and range of motion at the joint, producing combined clinical and mechanical symptoms [11, 12].
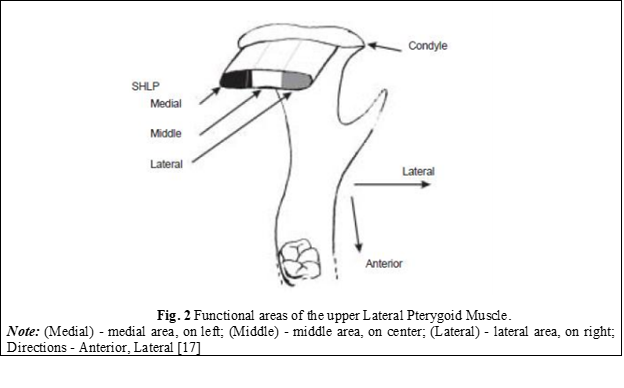
The functions of the lateral pterygoid muscle were defined as follows: the superior bundle is responsible for closing, retropulsion, and ipsilateral movements of the mandible, while the inferior bundle is active in opening, propulsion, and contralateral movements of the mandible. However, recent studies suggest that some fibers of the superior bundle may also be involved in opening, propulsion, and contralateral mandibular movements, and that it may consist of three mediolaterally arranged functional areas. This indicates that the concept that the occurrence of clicking, crackles in the TMJ is due to uncoordinated movements between both bundles of the lateral pterygoid muscle needs to be re-evaluated [10, 16]. Both fascicles of the lateral pterygoid muscle, in some recent reports, have been shown to be inactive during electromyography in the mandibular posture position. An important role of the lateral pterygoid muscle demonstrated electromyographically is that it generates laterality and propulsive movements of the mandible. This was observed when the activity of the inferior fasciculus changed when the direction of the horizontal force of the mandible oscillated from side to side [1, 10]. Murray et al. claim that the cause that would lead to different conclusions and results regarding the function of the lateral pterygoid muscle would be the incorrect placement of the electromyographic electrodes at the level of the muscle [17]. The results obtained could belong to other adjacent muscles such as the temporalis muscle or medial pterygoid muscle, or they could belong to the lateral pterygoid muscle (LPM) but be incorrectly assigned to a specific fascicle. With the help of computed tomography, they analyzed the correct placement of the electrodes at the level of the lateral pterygoid muscle and confirmed the classical notion that the inferior LPM would be involved in opening, protrusion, and contralateral mandibular movements. Studying the functional unit of the muscle, they concluded that the supero-medial part of the inferior LPM has an important role in initiating contralateral movements of the mandible, while the infero-medial part has a role in controlling fine movements [17]. The activity of the superior lateral pterygoid muscle was also confirmed by the correct placement of the electrodes, and it turned out to be much more complex than originally thought [17]. In contrast to the idea that the superior LPM is active only in closure, retrusion, and ipsilateral movements of the mandible, most muscle fibers of the superior LPM are at least active in opening, protrusion, and contralateral movements. Moreover, the pattern of activity varies depending on the location of the muscle fibers in the muscle and has been classified into three functional areas (Figure 2) [17]. The medial area produces patterns of activity similar to those produced by the inferior LPM. The lateral area may be equally active in closure, ipsilateral movements, and retrusion, while the central area generates different patterns of activity. These obtained functional data play an important role in understanding the role of the lateral pterygoid muscle in TMD [17].
The relationship between the lateral pterygoid muscle and the elements of the temporomandibular joint. J. Okeson described the functional importance of the superior lateral pterygoid muscle, namely: it becomes evident when the unilateral masticatory function is exercised. When an individual unilaterally bites a hard substance, both temporomandibular joints are functionally loaded differently. This occurs because the force is not applied to the joint but to the hard substance. Intra-articular pressure increases contralaterally to the biting area, and decreases at the ipsilateral articular level. This fact can lead to a separation between the articular surfaces, resulting in a dislocation of the articular disc on the same side. To prevent this, the superior lateral pterygoid muscle becomes active during biting, rotating the disc forward on the condylar surface so that the thicker edge of the disc remains in permanent contact with the articular surface [5].
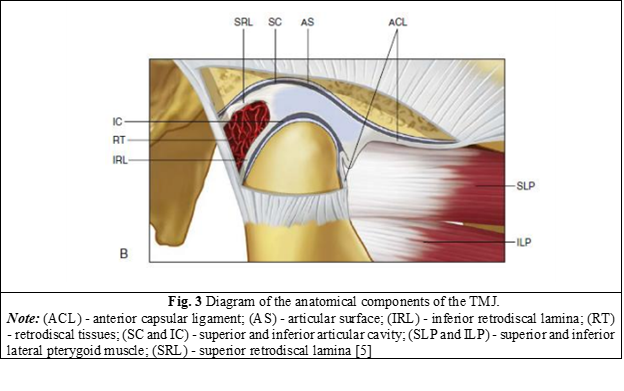
The articular disc is composed of several layers of collagen fibers, each oriented in different directions to resist the shearing phenomenon that could occur during the sliding of the condyle. The disc is attached to the medial and lateral surfaces of the condyle, which allows it to move in unison with the articular condyle. The positioning of the disc is controlled by a combination of elastic fibers attached to the posterior surface of the disc, thereby holding the disc in tension against the action of the superior fasciculus of the lateral pterygoid muscle, which is attached to the anterior surface of the disc. When the disc ligaments pull the disc along with the movement of the condyle, the rotation of the disc on the condyle occurs due to the force of contraction or relaxation of the superior fascicle of the muscle (Figure 3) [1, 4, 18].
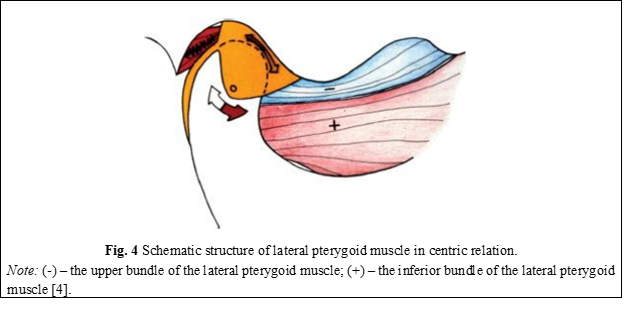
All the tissues attached to the disc have the role of preventing its anterior dislocation. However, the question arises as to how its anterior dislocation occurs, which is very common in patients with temporomandibular dysfunction. The only force that is directed anteriorly and can produce articular disc dislocation is the muscle that is attached to the anterior surface of the disc, namely the superior fascicle of the lateral pterygoid muscle. This muscle, together with the elastic collagen fibers attached posteriorly to the disc, controls the position of the disc on the surface of the condyle and is always aligned with the direction of the force when the condyle descends the slope of the tubercle downward [4]. If the articular condyle is located in centric relation, the articular disc is located in the most anterior position that the posterior ligaments allow. In this position, the condylar forces are directed upward toward the medial third of the disc and forward toward the anterior surface of the condyle. When the inferior fascicle of the lateral pterygoid muscle (+) begins to propel the condyle anteriorly, the superior fascicle of the muscle (-) relaxes its contraction to allow the elastic fibers to pull the disc over the condyle (Figure 4) [4].
At the maximum opening of the oral cavity, when the condyle reaches the most inclined portion of the articular tubercle, the disc should be directly located above the articular condyle, as the forces are directed superiorly. At this point, the elastic fibers rotate the disc posteriorly because the superior fasciculus of the lateral pterygoid muscle is in a controlled relaxation (Figure 5) [4].
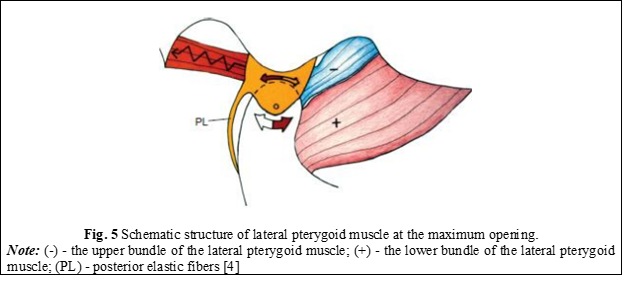
Upon closing the oral cavity, the condyle moves posteriorly and superiorly on the slope of the articular tubercle, so that the disc is again positioned in front of the condyle. For this, the upper fascicle (+) begins to contract, while the lower fascicle (-) relaxes and allows the condyle to return to its original position (Figure 6) [4].
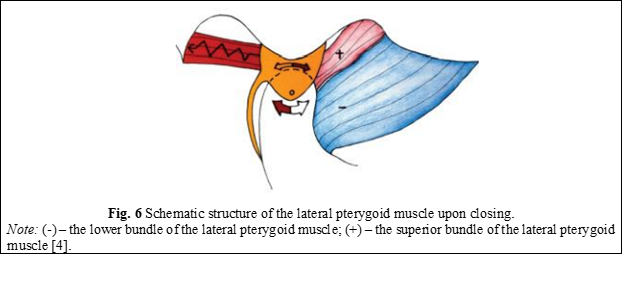
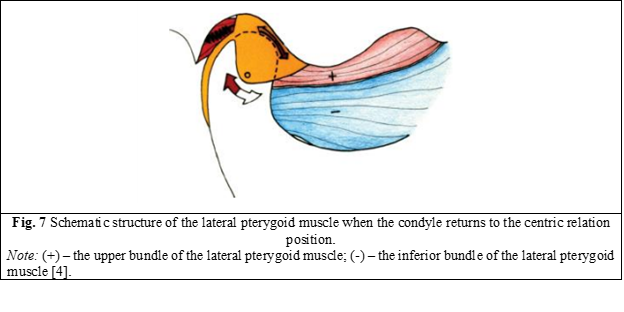
When the condyle returns to the centric relation position, the disc is located as anteriorly as possible, as far as the posterior ligament allows. If the ligament is intact and has not been stretched or torn, the disc is located in perfect alignment with the direction and position of the condyle. In the absence of occlusal interference, the inferior fascicle of the lateral pterygoid muscle is not active, even in the position of maximum intercuspation (MI). The superior fascicle remains contracted to keep the disc in the proper position (Figure 7) [4].
Correlation between anatomical variations of the lateral pterygoid muscle and temporomandibular dysfunctions.The correlation between the types of muscle insertions of the LPM and its anatomical variations is directly proven in studies of temporomandibular joint dysfunctions. Only two research studies from 2009 and 2012 (Dergin et al.; Matsunaga et al.) did not show any link between anatomical variations of the lateral pterygoid muscle and temporomandibular joint dysfunctions. This is because they represent studies carried out on corpses where only the morphological structure was studied and only the variations were observed [19, 20, 21]. The anatomical variation of muscle insertion type 2 according to the Litko classification [13] was the one that showed the closest connection with TMD, especially due to the insertion of the superior fasciculus of the LPM at the level of the articular disc and the articular condyle (Table 1). This variation can produce joint alterations or dysfunctions, characterized by local or loco-regional pain during closing or opening movements. However, studies have not shown any relationship between the type of insertion of the inferior fascicle of the LPM and TMD, both primary and secondary. The middle or accessory fascicle, which was described in 19% of cases in the analyzed studies, showed no connection between its presence and any TMD, primary or secondary, acute or chronic [21, 22-26]. Murray et al. state that observations made in recent years support the theory that the lack of coordination of movements between the upper and lower LPM could generate clicks, cracks, or articular blockages during the horizontal positioning of the disc in relation to the condyle. However, recent evidence indicates the possibility that fibers of the superior lateral pterygoid muscle that insert at the level of the articular disc could activate independently of other fibers of the same muscle, the superior LPM, and generate forces at the level of the disc that are not simultaneously generated with those inserted at the level of the condyle [17]. J. Okeson states that the exact percentage of insertion of the superior lateral pterygoid muscle at the level of the disc and at the level of the articular condyle is still under debate and is variable. However, it would be reasonable to assume that if the insertion of the muscle is predominant at the level of the neck of the articular condyle, and less at the level of the disc, the function of the superior muscle will have correspondingly less influence on the position of the articular disc. Moreover, vice versa, if the insertion of the superior muscle is predominant at the level of the articular disc, and less at the level of the condyle, the function of the muscle will greatly influence the position of the articular disc. This anatomical variation explains why, in some patients, the disc dislocates very quickly, without any antecedents or previous clinical features [5].
Conclusions
1. The lateral pterygoid muscle plays an obviously important role in the development of temporomandibular dysfunctions through the prism of its anatomical and functional particularities, specifically referring to the superior fascicle responsible for maintaining the correct anatomical position of the articular disc during function.
2. The anatomical variations of the muscle insertion according to various classifications are directly related to the development of TMJ dysfunctions, especially due to the insertion of the superior fascicle of the LPM at the level of the articular disc and the articular condyle. This variation can produce alterations at the level of the joint complex, characterized by the respective dysfunctional symptomatology. However, studies have not shown a clear link between the type of insertion of the inferior fascicle of the LPM and both primary and secondary TMJ dysfunction.
Competing interests
None declared.
Authors’ contribution
All the authors have contributed equally at the results presentation in the paper, approved the „ready for print” version of the manuscript.
Author’s ORCID IDs
Vitalie Pântea – https://orcid.org/0000-0002-3489-030X
Felicia Tabără – https://orcid.org/0009-0000-9979-8371
Mariana Ceban – https://orcid.org/0000-0001-7203-358X
Veronica Burduja – https://orcid.org/0009-0007-8101-9043
Lilian Nistor – https://orcid.org/0009-0008-6282-9240
Olga Ursu –https://orcid.org/0000-0002-2923-5546
References
Lai Y, Yap A, Turp J. Prevalence of temporomandibular disorders in patients seeking orthodontic treatment: a systematic review. J Oral Rehabil. 2020;47(2):270-280. doi: 10.1111/joor.12899.
Murphy MK, MacBarb RF, Wong ME, Athanasiou KA. Temporomandibular disorders: a review of etiology, clinical management, and tissue engineering strategies. Int J Oral Maxillofac Implants. 2013 Nov-Dec;28(6):e393-414. doi: 10.11607/jomi.te20.
Bernal C, Gonzalez O, Moreno M. New anatomo-radiological findings of the lateral pterygoid muscle. Surg Radiol Anat. 2016;38(9):1033-1043. doi: 10.1007/s00276-016-1665-2.
Dawson PE. Functional occlusion: from TMJ to smile design. St. Louis (Missouri): Mosby; 2007. 630 p.
Okeson JP. Management of temporomandibular disorders and occlusion. 8th ed. St. Louis (Missouri): Elsevier; 2020. 514 p.
Li D, Leung Y. Temporomandibular disorders: current concepts and controversies in diagnosis and management. Diagnostics (Basel). 2021;11(3):459. doi: 10.3390/diagnostics11030459.
Bordeniuc G. Indici clinico-fiziologici în disfuncția mușchilor masticatori [Clinical physiological index in masticator muscle disorder] [dissertation]. Chișinău: Nicolae Testemitanu State University of Medicine and Pharmacy; 2023. 159 p. Romanian.
Fala V. Implementarea design-ului funcțional-estetic, conform conceptului ocluzal „Ocluzia consecutivă cu dominanta canină” în terapia restaurativă estetică, metoda directă [Implementation of functional design, based on the occlusal concept “Sequential guidance with canine dominance” in aesthetic restorative therapy, through the direct method]. Chișinău: Sirius; 2020. 168 p. Romanian.
Sarlani E, Grace E, Reynolds M, Greenspan J. Evidence for up-regulated central nociceptive processing in patients with masticatory myofascial pain. J Orofac Pain. 2004;18(1):41-55.
Matsunaga K, Usui A, Yamaguchi K, Akita K. An anatomical study of the muscles that attach to the articular disc of the temporomandibular joint. Clin Anat. 2009;22(8):932-940. doi: 10.1002/ca.20865.
Antonopoulou M, Iatrou I, Paraschos A, Anagnostopoulou S. Variations of the attachment of the superior head of human lateral pterygoid muscle. J Craniomaxillofac Surg. 2013;41(6):91-97. doi: 10.1016/j.jcms.2012.11.021.
Valenzuela JJ, Orellana M, Gold M, Garcia G, Santana A. Anatomy of the lateral pterygoid muscle and its relationship with temporomandibular disorders: a literature review. Eur J Anat. 2020;24(3):249-256.
Litko M, Szkutnik J, Berger M, Rozyło-Kalinowska I. Correlation between the lateral pterygoid muscle attachment type and temporomandibular joint disc position in magnetic resonance imaging. Dentomaxillofac Radiol. 2016;45(8):20160229. doi: 101259/dmfr.20160229.
Kawakami S, Kodama N, Maeda N, Sakamoto S, Oki K, Yanagi Y, Asaumi J, Maeda T, Minagi S. Mechanomyographic activity in the human lateral pterygoid muscle during mandibular movement. J Neurosci Methods. 2012;203(1):157-162. doi: 10.1016/j.jneumeth.2011.09.026.
Omami G, Lurie A. Magnetic resonance imaging evaluation of discal attachement of superior head of lateral pterygoid muscle in individuals with symptomatic temporomanibular joint. Oral Surg Med Oral Pathol Oral Radiol. 2012; 114(5):650-657. doi.org/10.1016/j.oooo.2012.07.482.
Ruangsri S, Whittle T, Wanigaratne K, Murray GM. Functional activity of superior head of human lateral pterygoid muscle and isometric force. J Dent Res. 2005;84(6):548-553. doi: 10.1177/154405910508400612.
Murray GM, Phanachet I, Uchida S, Whittle T. The human lateral pterygoid muscle: a review of some experimental aspects and possible clinical relevance. Aust Dent J. 2004;49(1):2-8. doi: 10.1111/j.1834-7819.2004.tb00042.x.
Sritara S, Tsutsumi M, Fukino K, Matsumoto Y, Ono T, Akita K. Evaluating the morphological features of the lateral pterygoid insertion into the medial surface of the condylar process. Clin Exp Dent Res. 2021;7(2):219-225. doi: 10.1002/cre2.353.
Bhutada MK, Phanachet I, Whittle T, Peck CC, Murray GM. Regional properties of the superior head of human lateral pterygoid muscle. Eur J Oral Sci. 2008;116(6):518-524. doi: 10.1111/j.1600-0722.2008.00582.x.
Stöckle M, Fanghänel J, Knüttel H, Alamanos C, Behr M. The morphological variations of the lateral pterygoid muscle: a systematic review. Ann Anat. 2019;222:79-87. doi: 10.1016/j.aanat.2018.10.006.
Eberhard L, Giannakopoulos NN, Rohde S, Schmitter M. Temporomandibular joint (TMJ) disc position in patients with TMJ pain assessed by coronal MRI. Dentomaxillofac Radiol. 2013;42(6):20120199. doi: 10.1259/dmfr.20120199.
Dergin G, Kilic C, Gozneli R. Evaluating the correlation between the lateral pterygoid muscle attachment type and internal derangement of the temporomandibular joint with an emphasis on MR imaging findings. J Craniomaxillofac Surg. 2012;40(5):459-463. doi: 10.1016/j.jcms.2011.08.002.
Palla S, Farella M. Masticatory muscle pain. In: Mense S, Gerwin RD, editors. Muscle pain: diagnosis and treatment. Heidelberg: Springer; 2010. p. 193-227.
Phanachet I, Whittle T, Wanigaratne K, Murray GM. Functional properties of single motor units in inferior head of human lateral pterygoid muscle: task relations and thresholds. J Neurophysiol. 2001;86(5):2204-2218. doi: 10.1152/jn.2001.86.5.2204.
Phanachet I, Whittle T, Wanigaratne K, Murray GM. Functional properties of single motor units in the inferior head of human lateral pterygoid muscle: task firing rates. J Neurophysiol. 2002;88(2):751-760. doi: 10.1152/jn.2002.88.2.751.
Șcerbatiuc D, Iovu G. Disfuncțiile articulației temporo-mandibulare [Temporomandibular joint dysfunction]. Med Stomatol (Chisinau). 2014;31(2):13-19. Romanian.

