Introduction
Acute lower limb ischemia (ALI) is characterized by a sudden decrease in arterial perfusion of the pelvic extremity, potentially threatening the viability of the respective anatomical segment and requiring urgent evaluation and treatment [1]. ALI remains one of the most frequent vascular surgical emergencies, being associated with a high rate of amputation of the affected extremity and a mortality rate surpassed only by that recorded in cases of ruptured abdominal aortic aneurysm [2, 3].
Embolism, in-situ thrombosis of the native artery, stent or vascular graft thrombosis, arterial trauma, or a complicated peripheral aneurysm (sac thrombosis or distal embolization) stand among the common etiological factors of ALI [2, 4]. Atrial fibrillation and mural intracardiac thrombosis that develop after myocardial infarction are presently more frequently noted sources of peripheral embolism [4]. Concurrently, a significant proportion is attributed to iatrogenic embolism, which arises intraoperatively during percutaneous transluminal angioplasty (PTA) for peripheral arterial disease of the lower extremities – an endovascular intervention that is frequently performed in the daily practice of vascular surgery services [5]. In the same context, acute stent thrombosis, particularly at the level of the femoropopliteal artery, is diagnosed in over 6% of cases [6].
Restoring the patency of the arterial lumen as swiftly as possible, ideally within the initial 6-8 hours following the onset of ALI, is crucial for preserving the limb and constitutes the primary objective of treatment [4]. Conventionally, this is achieved through open surgery – thromb/embolectomy using the Fogarty balloon catheter. The technique is characterized by its surgical simplicity, cost-effectiveness, speed, and accessibility, and it is largely clinically effective, particularly in cases of embolic ALI where a single arterial segment is obstructed, especially above the knee [3, 7]. However, the same intervention doesn't yield a similar technical success in the presence of organized embolic masses situated within small-caliber arteries or when embolism occurs against the backdrop of peripheral arterial disease – a situation known as "acute-on-chronic" ischemia [7]. By the way, the latter is being increasingly registered in recent studies dedicated to peripheral arterial embolism [1]. Advancing the balloon catheter towards the infrapopliteal vessels, especially in diabetic patients where distal occlusive-stenotic lesions are characteristic, may encounter difficulties [8, 9]. Also, it's important to take into account that the procedure is typically carried out blindly in the vast majority of cases, without the option of separately guiding the catheter towards the lumen of each calf artery (tibial or peroneal arteries). The routine practice of performing fluoroscopic-assisted balloon thrombectomy or intraoperative angiography is still limited [3, 7, 8, 10]. In the clinical circumstances mentioned above, the extraction of thrombotic masses with the Fogarty catheter often remains incomplete, with the documented rate of residual thrombosis in small-caliber (distal) arteries varying between 36% and 86% [3].
Nonetheless, even in the absence of pre-existing atherosclerotic lesions, the indirect surgical embolectomy using a balloon catheter can be linked to the migration of thrombotic masses (resulting in distal embolization or, conversely, propagation in the proximal direction) or harm to the arterial wall (such as dissection or perforation), delayed pseudoaneurysm or arteriovenous fistula formation, and diffuse arterial narrowing due to intimal proliferation [5, 11]. Therefore, in cases of ALI, more intricate surgical interventions for limb revascularization might frequently be necessary: open procedures (like endarterectomy or bypass surgery) or hybrid approaches (combining open surgery with endovascular techniques) [12].
In an effort to address the aforementioned deficiencies in open surgical ALI treatment over the past two decades, specialized medical companies have introduced new technologies and devices for percutaneous revascularization. Consequently, the current array of endovascular treatment methods applicable to ALI patients encompasses: catheter-directed thrombolysis; ultrasound-accelerated thrombolysis; percutaneous mechanical thrombectomy involving rheolytic or fragmentation (rotational) techniques; pharmacomechanical thrombectomy; simultaneous angioplasty combined with thrombolytic irrigation (SATI technique); as well as manual percutaneous aspiration thrombectomy or the utilization of devices offering continuous automatic thromboaspiration [4, 7, 13]. To the latter group is also attributed the Penumbra Indigo® (Penumbra Inc., Alameda, CA, USA) – a device designed for extracting thrombotic masses/emboli from the lumen of peripheral vessels through percutaneous vacuum-assisted aspiration, applicable in cases of ALI and venous thrombosis. The initial data reported up to this point for the utilization of the Penumbra Indigo® system in ALI seem promising, but the overall evidence remains limited. The objective of the current study was to present the preliminary results of our initial experience with the application of percutaneous vacuum-assisted thromboaspiration in patients with non-traumatic ALI resulting from infrainguinal occlusions.
Material and methods
The study was carried out in the University Vascular Surgery Clinic, Chair of General Surgery-Semiology No.3 of the Nicolae Testemițanu State University of Medicine and Pharmacy (Department of Vascular Surgery, Institute of Emergency Medicine, Chișinău), during the period September 2022 – June 2023. Informed consent was acquired from all subjects encompassed by the study. The data from the electronic register of prospective records of patients who underwent revascularization surgery for ALI were evaluated, and cases treated with the application of percutaneous thromboaspiration using the Penumbra Indigo® system were selected for further analysis. The mentioned research received approval from the institutional Ethics Committee, within the project dedicated to the study of acute ischemia of the extremities (No.1 of 16.02.2021).
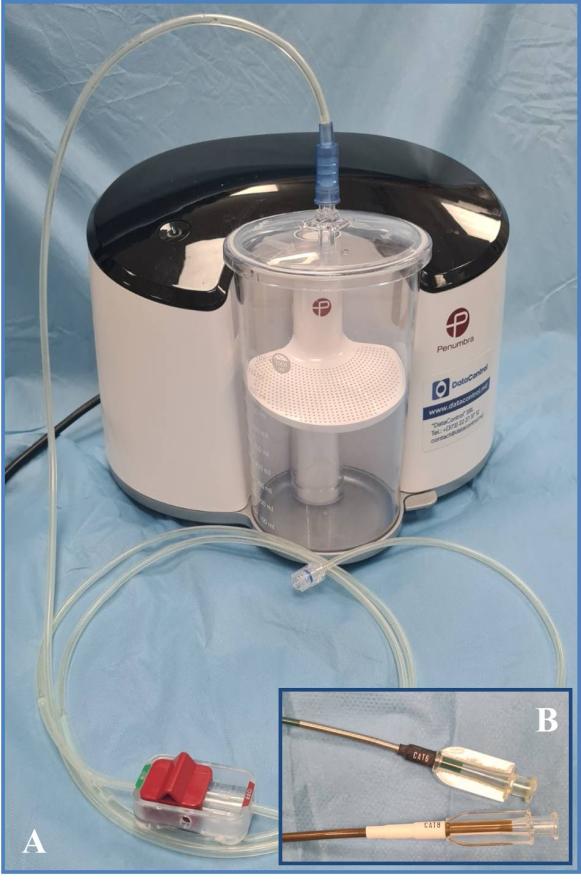
Features of Penumbra Indigo® system.As per the manufacturer's specifications, the Indigo® device (Fig. 1A) comprises the subsequent components: Penumbra Engine® aspiration source, Penumbra Engine canister, Indigo aspiration tubing equipped with a valve switch for system activation and deactivation, Indigo Separator™, and Indigo CAT™ mechanical thrombectomy catheters.
|
Fig. 1 General aspect of Penumbra Indigo® system (A) and the dedicated CAT6™ and CAT8™ mechanical thrombectomy catheters (B). |
The aspiration source is capable of providing a pure, continuous vacuum (-29 in Hg / 736.6 mm Hg / 98.2 kPa / 0.96 Atm), enabling the elimination of thrombi from the lumen of vessels with various diameters. This capability is also attributed to the availability of an extensive array of catheters with different diameters and lengths, designed to be tapered and resistant to collapsing (Fig. 1B): CAT3, CAT5, CAT6, CAT8 (including various tips: STR / TORQ / XTORQ), CAT D, CAT RX, or CAT7 and CAT12 – the latter two being of a newer generation.
Technical aspects of percutaneous vacuum-assisted thromboaspiration. Following the establishment of endovascular access and the placement of a 6F or 8F sheath, digital subtraction angiography (DSA) was conducted to confirm the location and extent of arterial occlusion. The guide-wire was advanced through the occlusive lesion, and subsequently, the dedicated thromboaspiration catheter was guided towards the proximal end of the lesion. After the catheter tip engaged the thrombus, the suction pump was activated, with a wait time of approximately 90 seconds to enable the creation of negative pressure. Subsequently, the suction tube switch was turned on, and the catheter was gradually withdrawn. Confirmation of thrombi aspiration was visual (by observing the presence of thrombotic masses in the canister after defoaming its contents), and also through DSA. In the presence of co-existing or underlying hemodynamically significant occlusive-stenotic lesions, their simultaneous endovascular treatment was applied, depending on the assessment of the operating surgeons.
Definitions and data interpretations. As per the guidelines, acute limb ischemia was defined with symptom duration less than 2 weeks [1]. The degree and clinical categories of ischemia were evaluated in line with the widely acknowledged Rutherford ALI classification system, incorporating criteria such as sensory loss, motor deficit, prognosis, and Doppler signals: gr. I (viable), gr. IIA (marginally threatened), gr. IIB (immediately threatened), gr. III (irreversible).
As primary endpoints of the study, the technical and clinical success of thromboaspiration were assessed, with secondary endpoints encompassing the rates of complications, primary patency, limb salvage, and 30-day mortality. Technical success was defined as restoring antegrade blood flow with near/complete aspiration of the embolus/thrombus and maintaining patency in at least one run-off vessel. For interpreting the technical results, the adapted classification for Thrombo-aspiration In Peripheral Ischaemia (TIPI), modified from the Thrombolysis in Myocardial Infarction (TIMI) classification, was employed [14]. Success was indicated by an increase of at least ≥1 point in comparison to the baseline score: 0 (no recanalization of the thrombotic occlusion), 1 (incomplete or partial recanalization of the thrombotic occlusion with no distal flow), 2 (incomplete or partial recanalization of the thrombotic occlusion with any distal flow), and 3 (complete recanalization of the thrombotic occlusion with normal distal flow).
Clinical success was defined as a post-interventional relief of ALI symptoms and an upward shift of at least one grade in the Rutherford classification. Complications/adverse events were categorized using the CIRSE classification system: gr.1 (can be resolved within the same session), gr.2 (requires prolonged observation <48 h), gr.3 (prolonged hospital stay >48 h or additional post-procedure therapy), gr.4 (causes permanent mild sequelae), gr.5 (causes permanent severe sequelae), and gr.6 (results in death) [15]. Primary patency was determined as a target lesion without haemodynamically significant stenosis (>50%) or re-occlusion on duplex scanning, conducted on the first postoperative day and at one month.
Continuous variables are presented as medians with interquartile range (25%-75%IQR), while categorical variables are represented as percentages.
Results
Patient data and ALI characteristics. The study group comprised 13 patients with ALI resulting from unilateral occlusion of the femoropopliteal arterial segment; 7 (53.8%) were males. Subjects' ages ranged from 43 years to 83 years, with a median value of 71 (25%-75%IQR 62.5-77.5) years. The right lower extremity was affected in 8 (61.5%) cases. All patients exhibited characteristic ALI symptoms (classic "6 P's"), occurring over 72 (25%-75%IQR 24-96) hours after onset (4-302). Thrombosis (n=9; 69.2%) or embolism (n=4; 30.7%) was identified as the etiological factor of ALI. According to the Rutherford classification, cases were distributed as follows: gr. I – 2 (15.3%), gr. IIA – 7 (53.8%), and gr. IIB – 4 (30.7%). In 10 (76.9%) instances, ischemia was categorized as "acute-on-chronic". Among comorbidities, the following were notable: arterial hypertension (n=13), chronic heart failure (n=13), ischemic heart disease (n=9), normo- (n=1) or tachysystolic (n=5) atrial fibrillation, diabetes mellitus (n=3), and chronic obstructive pulmonary disease (n=2). Each patient presented with at least three chronic diseases.
All patients were on chronic anticoagulant/antiplatelet treatment, while 6 (46.1%) were receiving antiarrhythmic medication. In 3 (23%) cases, a recurrent episode of ALI was identified, with the patients having undergone previous open embolectomy at the same limb level. Two (15.3%) patients had a history of superficial femoral artery stenting for chronic occlusive-stenotic lesions, and two others had undergone femoropopliteal below-knee autogenous vein bypass. Laboratory data revealed leukocytosis (n=6), hyperfibrinogenemia (n=6), mild anemia (n=4), and thrombocytopenia (n=2). All patients received therapeutic doses of anticoagulants upon admission: sodium heparin (n=10; 76.9%) or enoxaparin (n=3; 23%).
The occluded arterial segment determined by preoperative computer tomography angiography (n=4; 30.7%), duplex scan (n=5; 38.4%), or both examinations (n=4; 30.7%) included the superficial femoral artery (n=7) and popliteal artery (n=2). Thrombosis of the femoropopliteal below-knee bypass was identified in two patients, while two others experienced acute stent thrombosis of the superficial femoral artery.
Results of the application of thromboaspiration and adjuvant endovascular techniques.
Patients underwent revascularization through percutaneous mechanical thromboaspiration using the Penumbra/Indigo® system as a primary or salvage intervention within 9 (25%-75%IQR 2.5-48) hours after hospitalization (2-96). In 11 (84.6%) cases, thromboaspiration was performed with local anesthesia, and in two others – under spinal anesthesia. Endovascular access was established via the ipsilateral common femoral artery (n=10), crossover (n=2), or brachial artery (n=1). DSA before thromboaspiration revealed thrombotic occlusion with no distal flow (TIPI score = 0) in all instances. Thromboaspiration was conducted using dedicated CAT6™ (n=3) and CAT8™ (n=10) catheters as the initial choice, depending on the diameter of the targeted vessel. Technical success (TIPI score = 2-3) was achieved in 12 out of 13 (92.3%) cases. In one patient with bypass thrombosis, despite recanalization of the autologous venous conduit and subsequent PTA for infrapopliteal occlusive lesions, restoration of distal flow was not possible. Consequently, a decision was made to opt for catheter-guided thrombolysis.
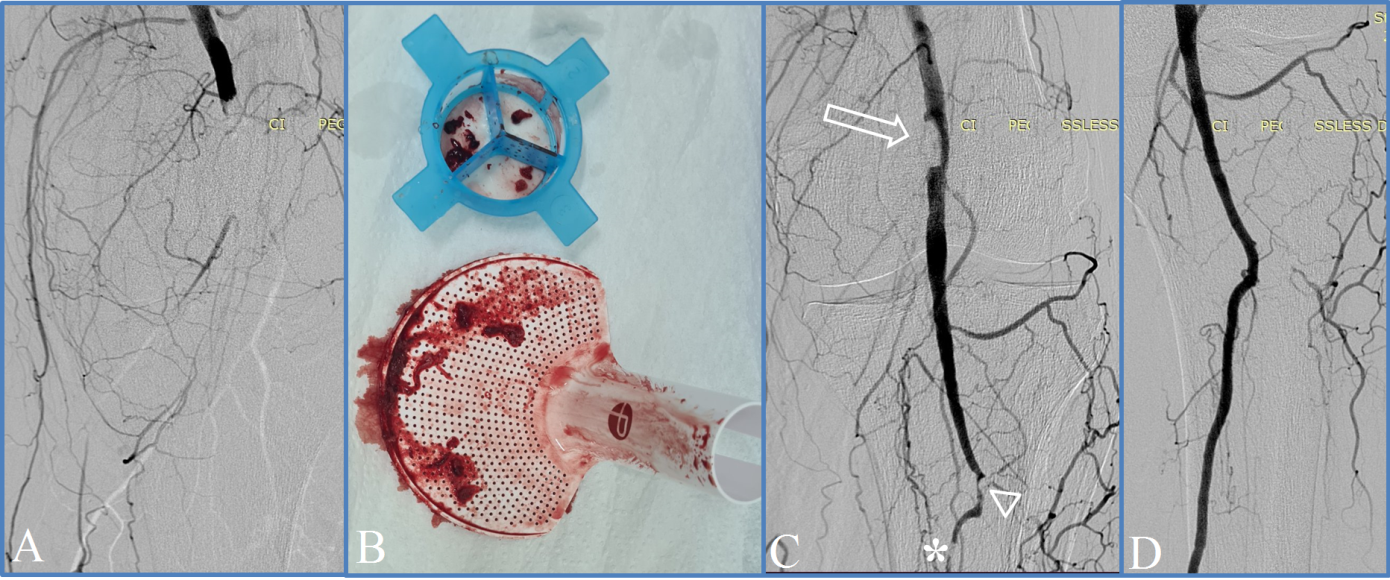
Control angiography following aspiration revealed associated infrainguinal occlusive-stenotic lesions in all cases, necessitating the use of adjunctive techniques: PTA, and in 8 (61.5%) cases – additional stenting of the femoropopliteal segment (Fig. 2). Distal embolization was identified during thromboaspiration in two cases, resulting in a perioperative complication rate of 15.3%. It's important to note that both instances were readily resolved during the same operative session through repeated aspiration (gr.1 CIRSE).
|
Fig. 2 Angiographic images captured during percutaneous thromboaspiration using the Penumbra/Indigo® system and the extracted thrombotic masses. Note: (a) – popliteal artery occlusion at diagnostic angiography; (b) – thrombotic masses aspirated into the canister of Indigo® device; (c) – persistent intraluminal embolus (arrow), intraoperative distal embolization (arrowhead) and concomitant chronic arterial lesion (asterisk) identified after initial thromboaspiration; (d) – restoration of blood flow at the completion angiography, after iterative aspiration followed by percutaneous transluminal angioplasty. |
The duration of surgical interventions ranged from 60 minutes to 160 minutes, with a median value of 120 (25%-75%IQR 72.5-120) minutes. The volume of intraoperative blood loss (aspirated into the canister) ranged from 260 ml to 480 ml. No patients required postprocedural blood transfusion. The clinical success rate was 92.3%. Follow-up duplex scanning confirmed the preservation of primary patency in all cases. There were no instances of death within the 30-day period, and the limb salvage rate was 100%.
Discussion
Despite its multiple shortcomings and potential perioperative complications, open thrombectomy with the Fogarty catheter remains the standard approach for ALI caused by embolism [1]. In contrast, the present study reflects the results of the initial experience of percutaneous vacuum-assisted thrombectomy using the Penumbra Indigo® system for ALI in the Republic of Moldova. In all four of our cases considered with embolic etiology, coexisting occlusive-stenotic lesions were identified after percutaneous thromboaspiration, necessitating adjunctive treatment – PTA or stenting. It can be assumed that in these cases, the standard approach might have overlooked the associated lesions with significant hemodynamic impairment, potentially affecting clinical outcomes or necessitating additional surgery. Moreover, three patients had previously undergone surgery for embolism, potentially exposing them to the associated risks of repeated procedures. Generally, considering the substantial percentage of cases presenting with "acute-on-chronic" ischemia (76.9%) and the dominance of arterial thrombosis (69.2%), the conventional open approach within the studied group likely would have necessitated complex revascularization interventions instead of simple balloon thromb-/embolectomy. Given the patients' advanced age and the elevated prevalence of comorbidities, we hold the view that bypass surgery might have been linked to notably higher morbidity rates compared to those observed following the application of the percutaneous technique.
The current guidelines suggest catheter-directed thrombolysis as an alternative to surgery in ALI, with both methods demonstrating comparable clinical outcomes [1, 3, 16]. However, thrombolysis has somewhat more limited indications, being recommended for less severe ALI cases (Rutherford grade IIA); and the rate of complications such as intracranial hemorrhage, major bleeding requiring surgery or transfusion, distal embolization, and compartment syndrome remains high (13%-30%) [11, 14, 17]. Furthermore, the approach demands meticulous monitoring in the intensive care unit with the requirement for subsequent angiography; it is time- and resource-intensive, and the reported high rate of technical success (up to 90%) can be attained only after the accumulation of extensive personal experience [16, 18]. Other previously mentioned endovascular techniques are also associated with a certain percentage of specific complications: hemorrhages, distal embolization (pharmaco-mechanical thrombectomy), hemolysis and renal failure (rheolytic thrombectomy), vessel injury (ultrasound-enhanced thrombolysis) [4, 19].
Among the endovascular techniques potentially associated with reduced periprocedural risks, percutaneous thromboaspiration stands out as a treatment option for ALI patients. In 1978, Horvath et al. first proposed the use of intra-arterial catheter aspiration to address iatrogenic embolism related to PTA [5]. Manual aspiration thrombectomy was subsequently described by Snidermann et al. in 1984 and successfully implemented using sheaths and catheters by Starck et al. in 1985 [11, 14, 20]. Traditionally, this procedure involves the use of a large-bore catheter and manual (50 ml) syringe aspiration, making it a cost-effective and widely available approach with a high technical success rate, ranging between 87% and 96% [21, 22]. Manual thromboaspiration has long been used as a complementary technique to thrombolysis, but current viewpoints suggest that it can also serve as the primary choice, with thrombolysis reserved for cases of treatment failure [10]. However, the method has several limitations. One of the primary concerns is the occurrence of sudden pressure changes during aspiration due to the inability of manual suction to maintain a stable (negative) pressure, which could potentially lead to distal embolization or the movement of the clot in a proximal direction [4, 7]. Additionally, the occurrence of arterial spasm, iatrogenic intimal dissections, or thrombosis is not negligible, mainly due to the necessity for multiple catheter movements [11, 20, 22].
Several devices for automatic thromboaspiration have recently been introduced, among which is the Penumbra Indigo® system. Initially, in 2005, the Penumbra mechanical thrombectomy system, utilizing vacuum aspiration as its primary mechanism of action, became available for revascularizing occluded intracranial vessels in patients with acute ischemic stroke [2, 14]. Following a successful initial experience in treating stroke, acute pulmonary embolism, and renovisceral occlusions, another device was launched in 2014 – the Penumbra Indigo® aspiration thrombectomy system, specifically designed for peripheral applications [5, 14]. The summarized early outcomes, reflecting the experiences of conducting peripheral thromboaspiration for ALI using the Penumbra Indigo® system across various medical centers, in addition to our own data, are outlined in Table 1.
Table 1. Concise synthesis of early outcomes of percutaneous thromboaspiration for ALI using the Penumbra Indigo® system from international experience. | |||
Author (year) | Number of cases | (Assisted) technical success rate | Limb salvage rate at 30 day |
Gandini et al. (2015) [8] | 3* | 100% | 100% |
Baumann et al. (2016) [18] | 33 | (53.9%)† | - |
Saxon et al.; PRISM trial (2017) [11] | 79‡ | (96.2%) | 97.5% |
Kwok et al. (2018) [19] | 15 | 53.3% | 100% |
Lopez et al. (2020) [16] | 43 | 51% | 88.4% |
Farhat-Sabet et al. (2020) [5] | 4 | 100% | 100% |
de Donato et al.; INDIAN trial (2021) [14] | 150 | 88.7% (95.3%) | 99.3% |
Zied et al. (2021) [21] | 19 | (94.7%) | 100% |
Rossi et al. (2021) [12] | 33 | 70% (90%) | 87.8% |
Present study (2023) | 13 | (92.3%) | 100% |
Note: *treated with Penumbra system (non-dedicated); †for above-the-knee occlusions; ‡52 cases – treated with Penumbra system (non-dedicated), 27 cases – treated with Indigo® system. | |||
Percutaneous aspiration thrombectomy is suitable for patients with anticipated difficulty during surgical embolectomy (morbidly obese, previous groin surgery) or an anticipated need for adjuvant endovascular procedures [21], as well as in cases when catheter-directed thrombolysis is contraindicated [11, 14]. As in the case of the application of many other techniques, the success of the method is determined by the selection of appropriate cases. More acute thrombus, presumably softer and more malleable, has a higher probability of successful removal [12, 18]. Despite the delayed referral of the patients in the current study (median value of ALI onset–presentation time being 72 hours), we obtained a high clinical success rate, presumably due to the predominance of atherothrombotic cases with well-developed collateralization.
The location of the arterial occlusion is considered a factor that can influence the technical success of the method. In the below-knee segments, the technique has a higher reported rate of success because the lesions are more often iatrogenic, shorter, and there is better concordance between the diameter of the vessel and the catheter [21]. Vice versa, a lower success rate in the above-knee lesions may be explained by a larger mismatch between the vessel size and the thrombectomy catheter size [18]. Therefore, the use of larger catheters, even in smaller vessels, is favored and believed to provide better thrombus removal [16].
Vacuum-assisted thromboaspiration minimizes the risk of endothelium injury and potential iatrogenic distal embolization, which are typical for open surgery [5, 11]. The reported rate of the latter during endovascular interventions is 1-5%, while in some studies it can be as high as 24% [22]. However, the actual frequency of this complication is believed to be much higher, estimated at 30-50%; fortunately, it often remains clinically silent [20, 22]. The open surgical approach in such cases, using the Fogarty balloon catheter in the below-knee arterial segment, could be ineffective due to the difficulty in directing the catheter into crural and foot arteries, and therefore does not appear to be an equivalent alternative to endovascular treatment [8]. In our study group, two (15.3%) cases of distal embolization were identified. However, it should be noted that these occurred during our initial practical experience when the full range of dedicated catheters was not yet available. Fortunately, both complications were ultimately resolved within the same intervention by repeating the aspiration procedure. Jung Guen Cha et al. reported a similar rate of distal embolization (16.7%). It's important to mention that the authors used a Penumbra aspiration catheter and a simple syringe instead of an automated device [3]. Overall, the rate of perioperative complications associated with the use of vacuum-assisted thromboaspiration is acceptable, ranging from 2% to 14%, being basically non-device specific [3, 14, 16].
Under the aspect of interventional technique, the use of access sheaths with removable check-flow valves that can be replaced, or rotating hemostatic valves, is considered beneficial. The last one allows for easier introduction of the catheters without damage to the tip, as well as removal of the clot intact when "corked" at the end of the aspiration catheter [11]. Even if its use remains at the discretion of the operating surgeon, the Indigo Separator™ allows thrombus disruption at the tip of the catheter ensuring its patency and makes possible fragmentation of the clot with cleaning the lumen without catheter removal [11, 14]. In general, it is believed that better results are related to a more precise technique, including the 1:1 sizing of the CAT™ catheter to the target vessel diameter in all cases, the application of the Separator™ in almost half of the cases, and the use of more than one catheter per case when necessary [14].
Judicious use of suction control by switching the on/off position of the tube valve and intermittent application of vacuum-assisted aspiration became important tricks for minimizing blood loss [11, 16]. By the way, the volume of hemorrhage in our study was comparable to the amounts described by other authors – on average 240 ml (up to 600 ml) [14].
To the disadvantages of percutaneous aspiration thrombectomy compared to catheter-directed thrombolysis can be attributed a potentially higher risk of traumatic injury to the endothelium and the inability to infuse lytic agents into collaterals or run-off arteries that are too small for an aspiration catheter [3]. However, it is important to mention that in the case of failure of vacuum-assisted thromboaspiration as an initial approach, the possibility of resorting to thrombolysis, embolectomy, or bypass surgery is not ruled out [14, 18]. In a similar context, unsuccessful open thromb-/embolectomy does not exclude the possibility of subsequent application of percutaneous thromboaspiration [3, 23].
Currently, other automated systems for percutaneous aspiration thrombectomy in ALI are also available, such as ClearLumen-II (ClearLumen-II, Groupates), which includes pulse spray thrombolysis; Aspirex (Straub Medical AG), which provides fragmentation of the thrombus by the spinning steel helix; or ThromCat XT (Spectranetics International, Leusden, The Netherlands), which implies rotational thrombectomy [7, 9]. More recently, the Indigo® Lightning™ 7 system, followed by the Lightning Bolt™ 7 and Lightning Flash™, have been implemented, providing intelligent computer-aided mechanical aspiration. Nevertheless, due to the limited evidence regarding the comparative efficacy of the respective methods, at the moment, it cannot be concluded that one of the thromboaspiration techniques is obviously superior in cases of ALI.
In conclusion, the Indigo® system represents a modern, minimally invasive technology with high effectiveness and a low rate of complications, suitable for both primary treatment of ALI and salvage or secondary therapy. The system setup is straightforward and doesn't necessitate the use of adjunctive devices or thrombolytic agents. Advantages of the technique include immediate restoration of blood flow and the absence of the need for protection filters. The availability of dedicated catheters with different diameters allows for reaching clots in very distal arteries, overcoming a limitation present in other techniques [8, 14]. The diverse clinical scenarios suitable for applying this technique to ALI patients, as also confirmed in our study (embolism, atherothrombosis, stent or bypass thrombosis, "acute-on-chronic" ischemia), highlight the wide applicability of the method. These factors could potentially contribute to a shift in the treatment trend for ALI in the near future.
Conclusions
Percutaneous vacuum-assisted thromboaspiration using the Penumbra/Indigo® system appears to be a safe and effective minimally invasive technique for treating ALI, providing the opportunity for concurrent correction of concomitant chronic peripheral arterial lesions.
Competing interests
None declared.
Patient consent
Obtained
Ethics approval
This study was approved by the Research Ethics Committee of Nicolae Testemițanu State University of Medicine and Pharmacy (minutes no. 1 of 16.02.2021).
Authors’ contributions
AP performed data collection and drafted the manuscript. VC participated in study design, performed statistical analysis, interpretation of data, and helped drafting the manuscript. DC conceived the study, interpreted the data and helped drafting the manuscript. All the authors reviewed the work critically and approved the final version of the manuscript.
Author’s ORCID IDs
Alexandru Predenciuc - https://orcid.org/0000-0002-2730-8115
Vasile Culiuc - https://orcid.org/0000-0003-3046-3914
Dumitru Casian - https://orcid.org/0000-0002-4823-0804
References
Björck M, Earnshaw JJ, Acosta S, Bastos Gonçalves F, Cochennec F, Debus ES, et al. Editor's choice - European Society for Vascular Surgery (ESVS) 2020 clinical practice guidelines on the management of acute limb ischaemia. Eur J Vasc Endovasc Surg. 2020;59(2):173-218. doi: 10.1016/j.ejvs.2019.09.006.
Lind B, Morcos O, Ferral H, Chen A, Aquisto T, Lee S, Lee CJ. Endovascular strategies in the management of acute limb ischemia. Vasc Specialist Int. 2019;35(1):4-9. doi: 10.5758/vsi.2019.35.1.4.
Cha JG, Kim CS, Kim YH, Kim SH. Effectiveness of percutaneous aspiration thrombectomy for acute or subacute thromboembolism in infrainguinal arteries. J Korean Soc Radiol. 2017;76(6):386-94. doi: 10.3348/jksr.2017.76.6.386.
Araujo ST, Moreno DH, Cacione DG. Percutaneous thrombectomy for initial management of acute limb ischaemia. Cochrane Database Syst Rev. 2019;(11):CD013486. doi: 10.1002/14651858.CD013486.
Farhat-Sabet AA, Tolaymat B, Voit A, Drucker CB, Santini-Dominguez R, Ucuzian AA, Toursavadkohi SA, Nagarsheth KH. Successful treatment of acute limb ischemia secondary to iatrogenic distal embolization using catheter directed aspiration thrombectomy. Front Surg. 2020;7:22. doi: 10.3389/fsurg.2020.00022.
Katsanos K, Al-Lamki SA, Parthipun A, Spiliopoulos S, Patel SD, Paraskevopoulos I, Zayed H, Diamantopoulos A. Peripheral stent thrombosis leading to acute limb ischemia and major amputation: incidence and risk factors in the aortoiliac and femoropopliteal arteries. Cardiovasc Intervent Radiol. 2017;40(3):351-9. doi: 10.1007/s00270-016-1513-0.
de Donato G, Pasqui E, Setacci F, Palasciano G, Nigi L, Fondelli C, Sterpetti A, Dotta F, Weber G, Setacci C. Acute on chronic limb ischemia: from surgical embolectomy and thrombolysis to endovascular options. Semin Vasc Surg. 2018;31(2-4):66-75. doi: 10.1053/j.semvascsurg.2018.12.008.
Gandini R, Merolla S, Chegai F, Del Giudice C, Stefanini M, Pampana E. Foot embolization during limb salvage procedures in critical limb ischemia patients successfully managed with mechanical thromboaspiration: a technical note. J Endovasc Ther. 2015;22(4):558-63. doi: 10.1177/1526602815589955.
Canyiğit M, Ateş ÖF, Sağlam MF, Yüce G. Clearlumen-II thrombectomy system for treatment of acute lower limb ischemia with underlying chronic occlusive disease. Diagn Interv Radiol. 2018;24(5):298-301. doi: 10.5152/dir.2018.18122.
Oğuzkurt L, Özkan U, Gümüş B, Coşkun İ, Koca N, Gülcan Ö. Percutaneous aspiration thrombectomy in the treatment of lower extremity thromboembolic occlusions. Diagn Interv Radiol. 2010;16:79-83. doi: 10.4261/1305-3825.DIR.2654-09.1.
Saxon RR, Benenati JF, Teigen C, Adams GL, Sewall LE; PRISM Trialists. Utility of a power aspiration-based extraction technique as an initial and secondary approach in the treatment of peripheral arterial thromboembolism: results of the multicenter PRISM trial. J Vasc Interv Radiol. 2018;29(1):92-100. doi: 10.1016/j.jvir.2017.08.019.
Rossi M, Tipaldi MA, Tagliaferro FB, Pisano A, Ronconi E, Lucertini E, Daffina J, Caruso D, Laghi A, Laurino F. Aspiration thrombectomy with the Indigo system for acute lower limb ischemia: preliminary experience and analysis of parameters affecting the outcome. Ann Vasc Surg. 2021;76:426-35. doi: 10.1016/j.avsg.2021.04.016.
Gupta R, Hennebry TA. Endovascular therapy for acute limb ischemia. Current and future trends in percutaneous intervention for limb salvage. Endovasc Today. 2010 Sept:90-95.
de Donato G, Pasqui E, Sponza M, Intrieri F, Spinazzola A, Silingardi R, Guzzardi G, Ruffino MA, Palasciano G, Setacci C; INDIAN trial collaborators. Safety and efficacy of vacuum assisted thrombo-aspiration in patients with acute lower limb ischaemia: the INDIAN trial. Eur J Vasc Endovasc Surg. 2021;61(5):820-8. doi: 10.1016/j.ejvs.2021.01.004.
Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. Cirse quality assurance document and standards for classification of complications: the Cirse classification system. Cardiovasc Intervent Radiol. 2017;40(8):1141-6. doi: 10.1007/s00270-017-1703-4.
Lopez R, Yamashita TS, Neisen M, Fleming M, Colglazier J, Oderich G, DeMartino R. Single-center experience with Indigo aspiration thrombectomy for acute lower limb ischemia. J Vasc Surg. 2020;72(1):226-32. doi: 10.1016/j.jvs.2019.10.079.
Rey E, Kaur S, Lee S, Arun G, Risam R, Hochberg K. A hybrid approach to acute limb ischemia utilizing Indigo aspiration thrombectomy in conjunction with catheter-directed thrombolysis. Vasc Dis Manag. 2022;19(2):E29-E33.
Baumann F, Sharpe E 3rd, Peña C, Samuels S, Benenati JF. Technical results of vacuum-assisted thrombectomy for arterial clot removal in patients with acute limb ischemia. J Vasc Interv Radiol. 2016;27(3):330-5. doi: 10.1016/j.jvir.2015.11.061.
Kwok CHR, Fleming S, Chan KKC, Tibballs J, Samuelson S, Ferguson J, Nadkarni S, Hockley JA, Jansen SJ. Aspiration thrombectomy versus conventional catheter-directed thrombolysis as first-line treatment for noniatrogenic acute lower limb ischemia. J Vasc Interv Radiol. 2018;29(5):607-13. doi: 10.1016/j.jvir.2017.11.030.
Schleder S, Diekmann M, Manke C, Heiss P. Percutaneous aspiration thrombectomy for the treatment of arterial thromboembolic occlusions following percutaneous transluminal angioplasty. Cardiovasc Intervent Radiol. 2015;38(1):60-4. doi: 10.1007/s00270-014-0857-6.
Zied S, Desoky H, Eldmarany H. Percutaneous-aspiration thrombectomy for acute and subacute lower-limb ischemia: feasibility and mid-term results. Egypt J Surg. 2021;40(4):1310-20.
Wei L, Zhu Y, Liu F, Zhang P, Li X, Zhao J, Lu H. Infrainguinal endovascular recanalization: Risk factors for arterial thromboembolic occlusions and efficacy of percutaneous aspiration thrombectomy. J Vasc Interv Radiol. 2016;27(3):322-9. doi: 10.1016/j.jvir.2015.11.025.
Papadoulas S, Kouri N, Mulita F, Katsanos K. Adjunctive vacuum-assisted aspiration thrombectomy in a patient with acute limb ischaemia and peronea arteria magna. BMJ Case Rep. 2021;14(8):e245490. doi: 10.1136/bcr-2021-245490.