Introduction
COVID-19 continues to pose a global public health threat. Epidemiological data indicate that patients suffering from metabolic disorders and chronic diseases are the most susceptible to SARS-CoV-2 (severe acute respiratory syndrome coronavirus). COVID-19 is currently considered a systemic disease with impaired immune system function that primarily affects the lungs, as well as the heart, kidneys, intestines, liver, and spleen. The particular effect of COVID-19 on the liver remains unclear, changes in liver biochemistry are common and occur in approximately 14 to 53% [1] of people infected with SARS-CoV-2. Liver biochemistry disorders are typically characterized by a moderate (1-2 times higher than normal) increase in serum levels of alanine aminotransferases (ALT) and aspartate aminotransferases (AST) [2, 3, 4]. In a study conducted in China, AST/ALT levels were elevated in 18.2/19.8% of patients with mild disease and in 39.4/28.1% of patients with severe disease [5]. Another small study in China showed similar results: elevated AST levels in 62% of patients admitted to the Intensive Care Unit (ICU) compared to 25%, who did not undergo treatment in the ICU [6]. In patients with a subclinical course of the disease, the AST and ALT levels were increased to 8.7 and 8.9% of patients, respectively [7]. The mechanisms of liver damage that occur during SARS-CoV-2 infection have not been clearly defined yet. The main pathogenetic consequences of liver damage include the following:
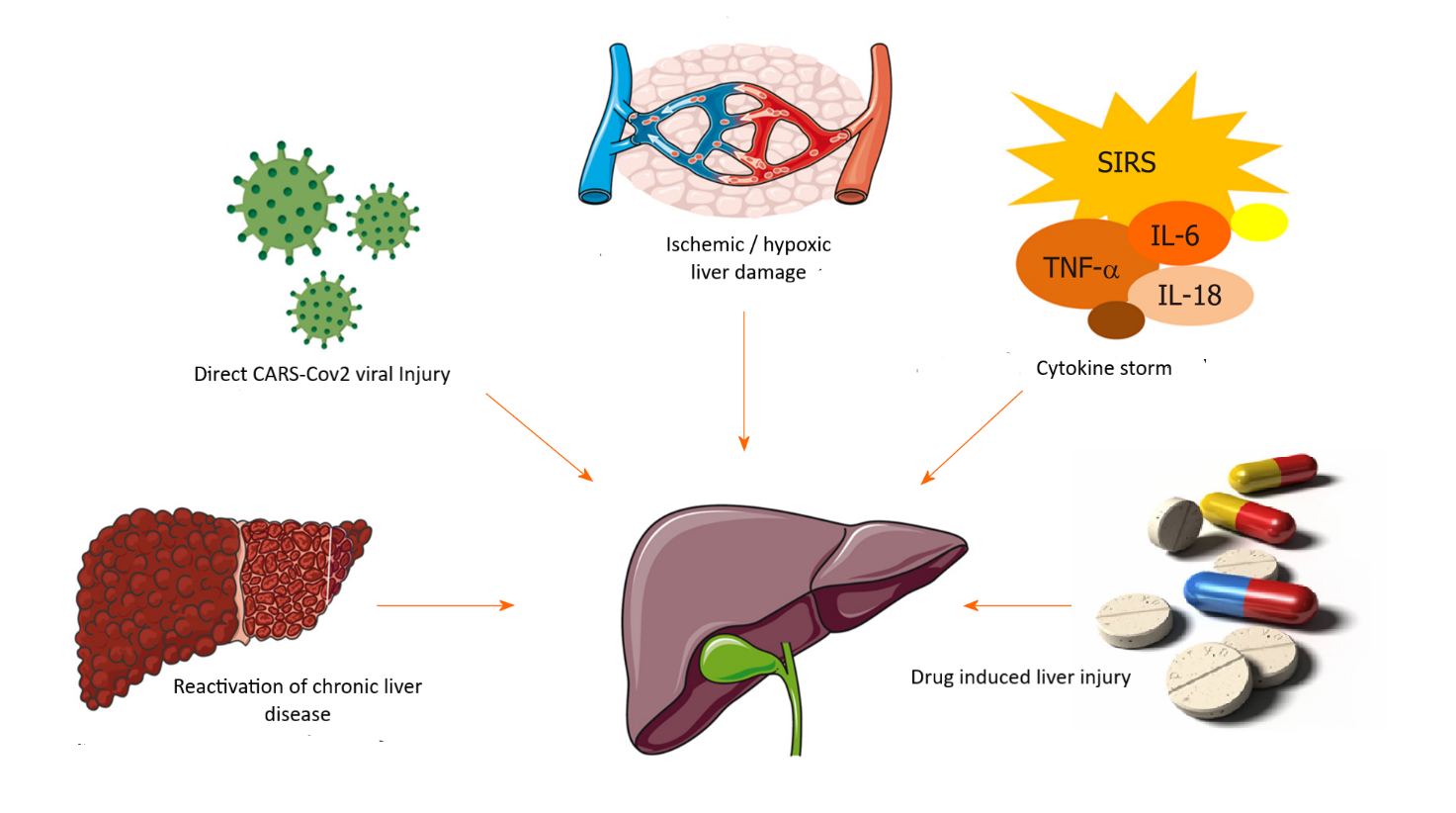
direct liver damage due to active replication of the virus in liver cells with the participation of ACE2 as receptors for introduction into the cell [8];
induction of severe hypoxia, which is considered one of the main causes of ischemic / hypoxic liver damage in COVID-19, followed by acute lung failure and / or shock. The liver damage is associated with metabolic acidosis, calcium overload, and changes in mitochondrial membrane permeability, being commonly manifested by cytolysis [9, 10];
immune activation and inflammation caused by circulating cytokines , followed by the cytokine „storm” onset and multiple organ failure [11, 12, 13];
direct hepatotoxicity of the administered drugs [5, 14];
reactivation of pre-existing chronic liver disease (such as chronic viral hepatitis B, C and E), as well as progression to decompensation of liver cirrhosis [1, 15].
The most common cause of liver damage in COVID-19 is associated with drug-induced toxicity resulting from the use of etiotropic treatment of SARS-CoV-2 infection according to approved clinical protocols and pathogenetic therapy for COVID-19 [5, 14]. Drug-induced toxic liver damage occurs in 10% of all drug-related adverse reactions [16]. There are many drugs with cumulative and hepatotoxic effects that can damage the liver. Some of them can cause an asymptomatic increase in liver enzymes, whereas in other cases, clinical signs may be characteristic of acute hepatitis. Liver damage may also be caused by the dose of the drug used (e.g. paracetamol overdose) or may not be dose-related. Liver toxicity can be induced by some drugs that can cause liver damage, such as antibiotics, anti-inflammatory drugs, and antivirals [17].
|
Fig. 1 Etiology of liver lesions in patients with COVID-19. (Source: Rui-Xu Y., Rui-Dan Z., Jian-Gao F. Etiology and management of liver injury in patients with COVID-19. In: J Gastroenterol 2020 Aug 28;26(32): 4753-4762, modified by the author). |
Paracetamol (acetaminophen). Paracetamol is the most common drug used as an antipyretic and anti-inflammatory drug, being used in medical practice by prescription and often as self-medication. Paracetamol is considered one of the most common hepatotoxic drugs with dose-dependent hepatotoxicity. The drug is safe if administered according to the dose, starting with body weight at one time or within 24 hours. It is necessary to consider the possibility of the synergistic effect when combining this drug with other ones, resulting in cumulative and hepatotoxicity effects. If the administered dose exceeds, the probability of developing drug-induced hepatitis sometimes is fulminant, followed by acute liver failure in 50% of pediatric patients [18].
It is the first antipyretic drug of choice in the treatment of coronavirus infection [19]. Undesirable side effects of paracetamol primarily include hepato- and nephrotoxicity. Toxicity is induced by the formation of metabolites derived from cysteine and mercapturic acid, which covalently bind to hepatocyte macromolecules, causing hepatocellular necrosis. When administering therapeutic paracetamol doses (10-15 mg/kg/ intake, not more than 60 mg/kg per day) the metabolite is inactivated by endogenous glutathione and excreted by the kidneys [20, 21]. Possibly, apart from the direct drug-induced hepatotoxicity, the occurrence of an idiosyncratic liver damage (immune- mediated) should be considered and identified separately, the most common cause being the antibiotic therapy [22].
Antimicrobial drugs Macrolides are widely used as antimicrobial drugs in children worldwide. Particular attention should be paid to monitoring their safety and harmfulness. According to the pharmacological properties of these drugs, almost all macrolides are hepatotoxic with varying degrees of severity. However, the doses used in clinical practice are insufficient for describing a direct hepatotoxic effect of macrolides. The adverse reactions commonly occur in presence of the following risk factors: high drug doses, genetic predisposition to hepatotoxicity, concomitant use of several drugs that foster the adverse effect of macrolides, as well as a present underlying liver disease. Special care should be given when prescribing potentially hepatotoxic drugs at highest doses, especially in pediatric patients. Cases of hepatotoxicity caused by azithromycin and clarithromycin have been reported in the scientific literature [23].
Anticoagulants may be one of the known causes of potential risk of liver damage [24]. Since the approval of sodium heparin by the Food and Drug Administration (FDA) in 1939, it is still widely used in medical practice for prevention and treatment of thrombosis[25]. The use of heparin has been associated with several well-documented side effects. The heparin-induced hepatotoxicity is less commonly recognized in clinical practice. Increases in AST and ALT levels have been frequently reported in the administration of both sodium heparin and low molecular weight heparins[26]. Previous clinical trials have shown that transaminase elevations occur in up to 90% of patients treated with heparin [27, 28]. An increase in the level of transaminases by more than 3 times from normal values indicates that it occurs in about 5% of patients receiving unfractionated heparin, and between 5% - 10% of patients administering low molecular weight heparins [29]. This increase in transaminase levels is usually temporary and self-limiting with no long-term effects.
Therefore, all patients receiving etiotropic and pathogenetic therapy for COVID-19 need to monitor liver parameters prior to following a treatment, throughout the treatment and in the post-COVID follow-up period to prevent severe drug-induced hepatitis in both active and post-COVID treatment.
Materials and methods
This article reports a clinical case study of a 12-year-old child, who was admitted to the Municipal Clinical Hospital for Children „Valentin Ignatenco” within the Covid-19 unit. The clinical and paraclinical data were retrieved from the inpatient medical records (lab findings included CBC, ALT, AST, total bilirubin and fractions, total protein, prothrombin index, fibrinogen, creatinine, urea, blood glucose, blood count, CRP, ferritin, D-dimers). According to medical records, the patient was confirmed with Covid-19 infection by performing a SARS-CoV-2 Rapid Antigen Test in the nasopharynx. Chest X-ray, abdomen ultrasound and check-ups at the neurologist, gastroenterologist-hepatologist, as well as at the infectious disease specialist were carried out.
Results and discussions
According to the medical history of the 12-years-old patient F., who was in contact with the mother infected with SARS-CoV-2, the child fell acutely ill on November 10 2021, experiencing severe malaise, an increase in body temperature up to 39°C, dry cough, sneezing, and rhinorrhoea. The mother self-administered antipyretics (ibuprofen combined with paracetamol) and antibiotics (Ceftriaxone intramuscular solution), which did not cause a clinical effect. Due to an increase in the malaise severity and the persistence of the febrile syndrome, the mother called an ambulance followed by urgent hospitalization on 10/20/21.
The patient has been monitored with severe psycho-verbal retardation and overweight since the age of one year. The objective clinical examination findings showed medium severity of the patient’s overall condition, including T - 38.5°C, RF - 20 r/min, RHR (resting heart rate) - 116 bpm, SpO2 - 97%, (SpO2 95% - 94% at 5-6 days, when acute bilateral pneumonia was confirmed) and BP - 120/80 mm Hg. Body weight - 67 kg, height - 147 cm, Percentile >97, BMI - 31 kg/m2 (+ 3DS). Pale and clean skin with no rash. No pathological picture of the mucous membranes and sclera, as well as a lacking peripheral lymphadenopathy was registered. Hyperemic pharyngeal isthmus. Free nasal breathing was observed. On auscultation, severe bilateral pulmonary respiration, with no rales was revealed. The heart sounds were clear and rhythmic. The abdomen was soft and painless to the touch; hepatomegaly +2.5-3 cm below the edge of the right costal arch, with a rounded edge, insensitive and painless, no ascites and splenomegaly. Physiological transit. Laboratory examination revealed the following data (table 1): leukocytosis - 15.88 x109/l (on the underlying bilateral pneumonia), neutrophilia - 72.2%, lymphopenia - 24%, increased ESR - 20 mm/h, elevated CRP >12.0 mg/l (on the underlying acute pneumonia), increase in ALT by 16 (50.9-487-764 U/l) compared to the normal references and AST 3 times higher than the norm (55.8 - 113 - 181 U/l), total bilirubin was normal, elevated fibrinogen - 5.3 g/l, ferritin - 2834 pmol/l and D-dimer levels - 762 ng/ml..
Table 1. Laboratory examination data in dynamics of patient F., a 12-year-old boy. | ||||||
Lab indices | Data | |||||
21.10.21 (Day 2) | 26.10.21 (Day 7) | 31.10.21 (Day 12) | 03.11.21 (Day 15) | 08.11.21 (Day 20) | 29.11.21 (after a 20-day follow up ) | |
Haemoglobin (N 110 - 120 g/l) | 131 | 127 | 146 | 148 | 136 | 138 |
Erythrocyte count (N 4.4 -5.0 x1012/l) | 4.96 | 4.91 | 5.57 | 5.62 | 5.15 | 4.12 |
Leucocyte count (N 4.0 – 9.0 x 109/l) | 6.10 | 7.35 | 15.88 | 14.94 | 7.04 | 7.15 |
Platelet count (180 - 400 x 109/l) | 226 | 211 | 397 | 351 | 217 | 382 |
Neutrophil count (N 43.0 – 60.0%) | 68.9 | 72.2 | 53.7 | 58.4 | 59.1 | 50.4 |
Lymphocyte count (N 30 – 46.0%) | 26.1 | 24.0 | 32.5 | 34.1 | 29.8 | 42.1 |
ESR (N 0 - 10 mm/h) | 19 | 20 | 14 | 16 | 32 | 23 |
CRP, mg/l | negative | >12,0 | negative | negative | >21,0 | negative |
Total protein (N36 - 60 g/l) | 60,1 | 61,6 | - | - | 69,9 | - |
Total bilirubin (8.5 – 20.5 mmol/l) | 4.45 | 4.72 | 6.87 | 6.08 | 12.72 | 6.4 |
AlAT (N 0 - 45 U/l) | 43.3 | 50.9 | 487.3 | 764.9 | 193.7 | 23 |
AST (N 0 - 45 U/l) | 43.2 | 55.8 | 113.,8 | 181.,6 | 61.7 | 21 |
Glucose (3.5 – 5.5 mmol/l) | 3.76 | 6.06 | 4.37 | 4.57 | 4.71 | 4.84 |
Urea (3.2 – 7.3 mmol/l) | 5.52 | 3.86 | 6.71 | 5.49 | 5.67 | - |
Creatinine (34 - 65 mmol/l) | 59,3 | 81,2 | 70,6 | 57,7 | 64,8 | - |
Prothrombin (95 - 105 %) | 97 | 99 | 95 | - | 82 | - |
Fibrinogen (1.25 - 4 g/l) | 4.0 | 5.3 | 3.1 | - | .8 | - |
INR | 1.03 | 1.01 | 1.05 | - | 1.22 | - |
Ferritin, pmol/l (N 61.6 - 803) | - | 2834 | - | - | 853 |
|
D-Dimer, ng/ml (N 100 - 500) | - | 762 | - | - |
| 0.15 |
Note: N - normal reference value; ESR - Erythrocyte Sedimentation rate; CRP - C-reactive protein; AlAT - Alanine aminotransferase; AST - Aspartate aminotransferase; INR - International Normalized Ratio. | ||||||
The chest X-ray revealed signs characteristic of right pneumonia, on day 5 of hospital stay, and negative radiological dynamics resulted in a bilateral pneumonia, 6-score Brexia, and cardiomegaly on day 11 of hospitalization. The abdominal ultrasound findings were suggestive for an inflammatory process in the liver, pancreas and kidneys; the liver was enlarged, RL = 134 mm, LS = 90 mm, portal vein = 7 mm with regular, clear contour, homogeneous parenchymal structure, increased echogenicity; gallbladder: atypical shape, fundal bend, dimensions 82 x 36 mm, perennials not thickened, no calculi; choledochus was not thickened; pancreas: increased echogenicity, homogeneous parenchymal echostructure, dimensions 17 x 16 x 22 mm, with regular contour, thickened; enlarged spleen with normal echogenicity, homogeneous parenchymal echostructure, dimensions 127 x 46 mm, regular contour; kidneys showed regular, clear contour, pelvises were not dilated, the calyx system was bilaterally deformed; bladder with little content, the walls were not thickened. An accurate diagnosis was carried out, including blood tests negative for HBsAg, negative total anti-HBcor, and negative anti-HCV. Covid-19 infection was confirmed by the SARS-CoV-2 Rapid Antigen Test of the nasopharyngeal exudate. The patient was consulted multidisciplinary for infectious diseases, by the gastroenterologist - hepatologist, pulmonologist and neurologist. Based on the objective examination data, as well as on the laboratory and instrumental findings, the following clinical diagnosis was established: moderate-form COVID-19 infection with bilateral pneumonia, acute evolution, severe-to-moderate form. Type 1 acute respiratory failure. Third-degree toxic hepatitis (based on RUCAM score - 8 points). Severe psycho-verbal retardation. Autism? First-degree acquired obesity (BMI - 31 kg/m2). The child received treatment according to the recommendations provided by the national and international pediatric medical protocols and standards. Antiviral treatment (Pranogir tab.); intensive symptomatic treatment: antipyretics (Paracetamol tab. 500 mg x 3 times/daily, per oral, at temperature ≥38.5°C); on first 4 days of hospitalization, bronchodilators (Salbutamol aerosol), probiotics (Subtil caps.), proton pump inhibitors (omeprazole caps., vitamin therapy). Considering the persistence of fever, the elevated CRP levels and the negative dynamics of the chest X-ray (acute pneumonia), a glucocorticoid therapy was administered to reduce the inflammatory response, namely low-dose Dexamethasone 0.2 mg/kg/day for 5 days and antibacterial treatment with macrolides in combination with third-generation cephalosporin (Ceftriaxon intravenous, Clarithromycin per oral). For the prevention of venous thromboembolism, basic anticoagulant therapy was carried out viz. Heparin 100 IU/kg/day subcutaneously for 9 days under coagulation testing. During treatment, intoxication syndrome, febrile syndrome, hypercoagulability, acute pneumonia and respiratory failure (SpO2 98%) regressed. Another significant challenge arising on the underlying treatment was the development of cytolysis syndrome (table 1), most likely associated with the use of the following drugs: paracetamol, heparin, and clarithromycin. Moreover, the unfavorable underlying conditions should also be considered, such as the first-degree obesity and long-term steatosis (steatohepatitis), thus referring the patient F. to a possible risk group for drug hepatotoxicity. Hepatoprotectors like Silymarin tab. per oral, then Essentials caps. per oral, and Aminoplasmal Hepa intravenously were administered. The child was discharged with improved clinical and paraclinical features, being indicated to follow the oral therapy with hepatoprotectors, probiotics, enzyme and vitamin therapy under the control of hepatic transaminases, coagulation and abdominal ultrasound.
Conclusions
The upper digestive system in children is the „gateway” for many disease-casing agents, including the coronavirus. The SARS-VOC-2 virus enters the body through the faecal-oral route, attaching to the epithelial cells of the mucous membrane of the upper respiratory and digestive tract, and subsequently spreading to internal tissues and organs. The interaction of coronavirus with the digestive tract of a child may lead to consequences, sometimes by a long-term elimination of SARS-CoV-2 through feces. In some patients, nausea, vomiting, abdominal pain, diarrhea, and liver damage may be associated with respiratory manifestations. If chronic diseases of the digestive system (cholecystitis, pancreatitis) are recorded in a pediatric patient, they can be reactivated, clinically showing both respiratory signs and digestive manifestations characteristic of a flare-up period of a chronic disease. Apart from the toxic effect of coronavirus on the liver and gastrointestinal tract, patients with COVID-19 also might have frequent and sometimes toxic side effects due to the complex treatment, as well as synergism due to drug overlapping. Antibacterial, antiviral, anticoagulant and drugs, especially those with hepatotoxic effects, can cause toxic side effects due to an overlapping mechanism, inducing toxic hepatitis, colitis, toxic biliary sludge the phenomenon. Therapeutic approach for Covid-19 infection in children should be individually-customized based on clinical indications and dynamic monitoring of the clinical disease evolution. Although liver damage may be associated with the viral direct cytopathic effects, the mechanism of liver damage during SARS-CoV-2 infection requires further in-depth scientific study.
Competing interests
None declared
Authors' contributions
The authors contributed equally to the search of scientific literature, the selection of bibliography, the reading and analysis of biographical references, the writing of the manuscript and its peer review. All authors read and approved the final version of the article.
Authors’ ORCID IDs
Tatiana Raba - https://orcid.org/0000-0002-3970-6495
Lucia Ciobanu - https://orcid.org/0000-0002-3583-4051
References
Zhang C., Shi L., Wang F. Liver injury in COVID-19: management and challenges. Lancet Gastroenterojgy & Hepatology, 2020; 5 (5): 428-430.
Sultan S., Altayar O., M Siddigue S. et al. AGA institute rapid review of the gastrointestinal and liver manifestations of COVID-19, meta-analysis of international data, and recommendations for the consultative management of patients with COVID-19. Gastroenterology, 2020; 159 (27): 320-334.
Richardson S., Jamie S., Narasimhan M. et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA, 2020; 323(20): 2052-2059.
Goyal P., Choi J., Pinheiro L. et al. Clinical characteristics of Covid-19 in New York city. N. Engl. J. Med. 2020; 382: 2372-2374.
Guan W-J, Ni Z-Y, Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708-1720.
Huang C., Wang Y., Li X. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395: 497-506.
Shi H., Han X., Jiang N. et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan China: a descriptive study. Lancet Infect Dis. 2020; 20: 425 - 434.
Hamming I., Timens W., Bulthuis M.L. et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004; 203(2): 631 – 637.
Xu L., Liu J., Lu M. et al. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020; 40(5): 998 - 1004.
Gupta A., Madhavan M.V., Sehgal K. et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020; 26:1017–1032.
Mehta P., McAuley D. F., Brown M. et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020; 395(10229): 1033 – 1034.
Wong CK., Lam CW., Wu AK. et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004; 136(1): 95-103.
Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323(11): 1061-1069.
Li J., Fan J.G. Characteristics and mechanism of liver injury in 2019 coronavirus disease. J. Clin. Transl. Hepatol. 2020; 8(1): 13-17.
Zippi M., Fiorino S., Occhigrossi G., et al. Hypertransaminasemia in the course of infection with SARS-CoV-2: incidence and pathogenetic hypothesis. World J. Clin. Cases. 2020; 8.(8): 1385 – 1390.
Ивашкин В.Т. (ред.). Болезни печени и желчевыводящих путей. М.: Издательский дом «М-Вести». 2002. 416 c. [Ivashkin V.T. (ed.) Diseases of the liver and biliary tract. M.: Publishing house “M-Vesti”, 2002. 416 p. (In Rus.)].
Cascella M., Rajnik M., Cuomo A. et al. Features, evaluation, and treatment of coronavirus. [Updated 2020 Oct 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2020.
EASL 2019. Clinical Practice Guidelines: Drug-induced liver injury. In: J Hepatol, 2019; 70(6): 1222 - 1261.
Временные методические рекомендации. Профилактика, диагностика и лечение новой коронавирусной инфекции (COVID-19). Версия 5 (08.04.2020). http://nasci.ru/?id=10642&download=1.
Маркова И.В., Афанасьев В.В., Цыбулькин Э.К. Клиническая токсикология детей и подростков. В 2-х томах. Т. 1. СПб.: Интермедика. 1998. 302 с. [ Markova I.V., Afanasyev V.V., Tsybulkin E.K., et al. Clinical toxicology of children and adolescents: in 2 volumes. T. 1. SPb .Intermedika. 1998; p. 302].
Bessems J., Vermeulen N. Paracetamol (acetaminophen)-induced toxicity: molecular and biochemical mechanisms, analogues and prote. Crit Rev Toxicol.2001; 31(1): 55 - 138.
Звягинцева Т.Д., Чернобай А.И. Лекарственные поражения печени. НПВП-ассоциированные гепатопатии: актуальность проблемы и современные терапевтические подходы. Український медичний часопис. 2014; 1: 80-85. http://nbuv.gov.ua/UJRN/UMCh_2014_1_16.
Белоусов Ю., Лекарственные поражения печени, ассоциируемые с макролидами. Очевидна ли связь? РМЖ; 2011; 18: 1118 – 1121.
Mahamid M., Mader R., Safadi R. Hepatotoxicity of tocilizumab and anakinra in rheumatoid arthritis: management decisions. Clin Pharmacol. 2011; 3: 39 - 43.
Center for drug evaluation and research. Summary review. Heparin Sodium. Data of submission 11.04.2011. Verified 11.11.2018. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/201370Orig1s000….
Bounameaux H. Unfractionated versus low-molecular-weight heparin in the treatment of venous thromboembolism. Vasc Med. 1998; 3(1): 41 - 46.
Bosco M., Kish T. Hepatotoxicity With Elevated Bilirubin Secondary to Prophylactic Doses of Unfractionated Heparin: A Case Report and Review of Heparin-Induced Hepatotoxicity. J Pharm Technol. 2019; 35(1): 36–40.
Harrill AH., Roach J., Fier I. et al. The effects of heparins on the liver: application of mechanistic serum biomarkers in a randomized study in healthy volunteers. Clin Pharmacol Ther. 2012; 92:214-220.
Arora N., Goldhaber S., Anticoagulants and transaminase elevation. Circulation. 2006; 113 (15): 698-702.