Introduction
While the incidence of deep infiltrating endometriosis (DIE) continues to rise, the pathogenesis, clinical manifestations, diagnosis, and treatment of this pathology remain subjects of intensive research worldwide.
DIE is considered the most aggressive and painful form of pelvic endometriosis, constituting one-fourth of its three phenotypes [1]. Affects 2% of women of reproductive age, DIE is responsible for pelvic pain syndrome, complicated somatic and surgical anamnesis, and a reduced quality of life for these women [2, 3]. Its diagnosis and treatment pose an extremely challenging task for both the global medical community and healthcare professionals in the Republic of Moldova.
Although DIE was first described by C. Rokitansky in 1860, only in 2012 Koninckx proposed the first definition of this pathology, stating that DIE is the invasion of endometrial tissue to a depth of more than 5 mm beneath the peritoneum [4]. In 2015, the DIE lesions were discovered in the intestines, bladder, ureters, and diaphragm. In 2017, Balle and Dara proposed the modern definition of the DIE.
Thus, DIE is the pathology that involves fibromuscular infiltration of the organs and anatomical structures with the subperitoneal invasion of the endometrial tissues, regardless of the depth of infiltration [4, 5].
For clinical use in 2021, the following classifications were recommended: the revised American Society for Reproductive Medicine (rASRM), the Endometriosis Fertility Index (EFI), and the #Enzian classification. These classifications allow staging the pathological process and assessing the reproductive prognosis for each patient [6, 7].
Of particular interest for our study is the #Enzian classification, reflecting various locations of DIE [8]. Some studies suggest that this classification correlates with the clinical manifestations of DIE; however, randomized studies are needed for definitive conclusions. From a clinical standpoint, this unified reporting system simplifies the medical management of patients, avoiding multiple repeat surgeries and improving the quality of treatment and quality of life for DIE patients [8].
DIE most commonly affects the patients under 17 years old, with 20% of girls manifest the pathology simultaneously with menarche. According to the literature, the clinical manifestations of DIE include the 4 "D" symptoms: dysmenorrhea, dyspareunia, dysuria, and dyschezia, often in combination with infertility [9]. DIE is associated with infertility, primarily due to the distortion of normal pelvic organ anatomy. The intensity of pelvic pain syndrome in DIE often exceeds 6 points on the VAS (visual analog scale), and extragenital foci provoke the development of symptoms with a catamenial course, such as pain during defecation, intestinal obstruction syndrome, pain during urination, hematochezia and hematuria, recurrent cystitis, chest pain, pain in the operation scar with cyclic bleeding and gradual formation increase in this area, and others [10-15].
Based on the above information, it can be argued that DIE is the most aggressive and clinically vivid phenotype of endometriosis, significantly deteriorating the quality of life for the patients [16, 17].
Infiltration of DIE foci into adjacent organs increases the frequency of interventions with surgical and anesthetic risks, further diminishing the quality of women's lives [17]. Currently, evaluating the quality of life for patients is an essential element of the medical care, and studying the determinants of quality of life helps better understand the specific impact of a particular disease on the patients' well-being [18]. Research reports indicate that the work productivity of women with DIE is reduced by 38%, 50% of patients suffer from infertility, and 88% of patients experience anxiety disorders or depression [19]. International guidelines recommend initiating studies dedicated to examining the quality of life of women with endometriosis using specialized questionnaires (SF36, EIQ, etc.) that reflect all levels of patients' health [4].
Having reviewed the above information, it was decided to conduct a comparative clinical study aimed at investigating the diagnostic features of DIE and determining its impact on the quality of life of patients to optimize its preoperative diagnostics.
Material and methods
A single-center cohort clinical study was conducted over 2 years at the Gheorghe Paladi Municipal Clinical Hospital. The study was approved by the Research Ethics Committee of Nicolae Testemiţanu State University of Medicine and Pharmacy (minutes nr. 38, dated 21.05.2021). The study included reproductive-aged patients diagnosed with "Endometriosis," confirmed based on intraoperative findings or ultrasound/MRI indications, who consented to participate in this study. The exclusion criteria for this study were as follows: patients under the age of majority, virgin patients, retired patients, patients with endometriosis malignancy, patients with severe extragenital pathologies (hypertension, cardiovascular pathology, liver pathology, and others), patients with precancerous or cancerous conditions (cervical, endometrial, ovarian), patients who refused to participate in the clinical study. Each patient signed an informed consent form to participate in this study.
Thus, the 190 women enrolled in the study were divided into two groups: the study group comprised 85 patients with deep endometriosis, while the control group consisted of 105 women with other forms of endometriosis (the ovarian endometriomas and superficial endometriosis).
To objectify the pain syndrome, the Visual Analog Scale (VAS) and Biberoglu and Behrman (B&B) pain scales were used, categorizing pain as "mild," "moderate," "severe," and "very severe." Intraoperative data and protocol data from paraclinical studies were analyzed with the staging of endometrioid processes according to the #Enzian classification. To assess the impact of endometriosis on the quality of life, three questionnaires were utilized: the Endometriosis Impact Questionnaire (EIQ), the 36-Item Short Form Health Survey (SF-36), which identifies the impact of endometriosis on 8 determinants of quality of life, and the World Health Organization-Five Well-Being Index (WHO-5), which evaluates the psychological well-being of patients. Analyzed data were recorded in an Excel spreadsheet, and statistical calculations were performed using the SPSS program. For comparing quantitative variables in groups, the Mann-Whitney U test was utilized. For comparing qualitative variables in groups, the Pearson's Chi-square test (χ²) was applied.
Results
Gynecological anamnesis data. Statistical comparison did not reveal any age difference between the patient groups (U = 4520.00, p = 0.879). The average age of women in the main study group was 32.39±0.81 years (95% CI [30.76 – 34.02 years]), while in the control group, it was 30.26±0.66 years (95% CI [28.91 – 31.60 years]). However, a significant difference in the age of onset of menarche was found between the study groups (U = 5697.00, p = 0.001), indicating an earlier manifestation of menstrual function among patients with deep infiltrating endometriosis (12.27±0.19 years; 95% CI [11.89 – 12.65 years]) compared to women with superficial and ovarian forms (13.18±0.20 years; 95% CI [12.77 – 13.59 years]). Statistical comparison of menstrual cycle regularity in the research groups revealed a significant difference (χ² = 11.206a, df = 1, p = 0.001), indicating a higher frequency of irregularities among women with deep endometriosis (40.0±5.4%; 95% CI [29.3 – 51.1%]) than those with other phenotypes of this pathology (18.1±5.4%; 95% CI [11.5 – 26.2%]). No statistically significant difference in menstruation duration was found between the research groups (U = 3840.50, p = 0.086), with a median of 5 days in both groups.
Medical history of patients. An analysis of the collected data revealed that in the main study group, patients' medical history was uncomplicated for only 16.5±4.1% (95% CI [8.6 – 25.3%]), whereas in the control group, this figure was 71.4±4.3% (95% CI [62.4 – 79.4%]), indicating a statistically significant differences (χ² = 81.844a, df = 6, p < 0.01) and indirectly suggesting reduced quality of life in women with deep endometriosis.
The time to diagnosis for patients with deep endometriosis exceeded 10 years in 67.1±5.3% (95% CI [56.5 – 76.7%]), while in the control group, the diagnosis was correctly made within 1 year of the disease in 56.2±4.9% (95% CI [46.3 – 65.6%]) of patients, indicating a statistically significant difference (χ² = 112.487a, df = 3, p < 0.01). This fact indirectly confirms the reduced quality of life in women with deep endometriosis.
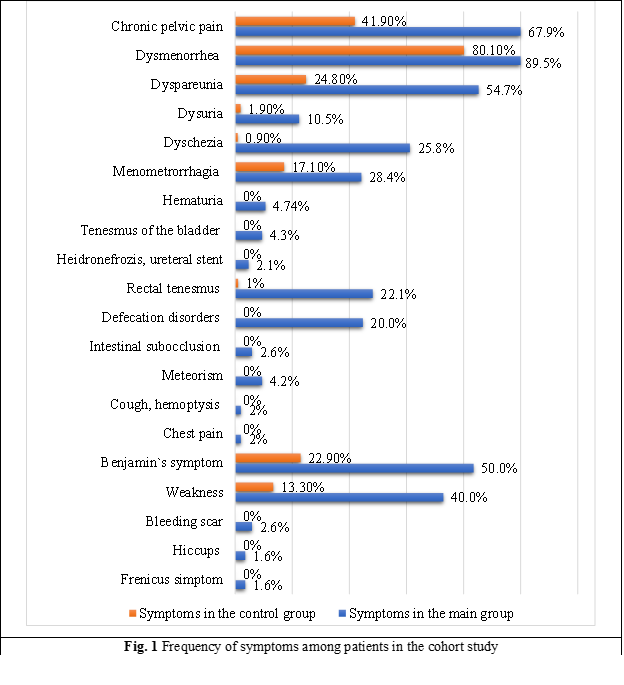
Endometriosis symptoms. The results of assessing complaints from patients in this study showed the following frequency of symptoms: chronic pelvic pain – 67.89%, dysmenorrhea – 89.47%, dyspareunia – 54.74%, dysuria – 10.53%, dyschezia – 25.79%, menometrorrhagia – 28.42%, catamenial hematuria – 4.74%, catamenial tenesmus of the bladder – 4.21%, hydronephrosis, ureteral stenting during pregnancy – 2.11%, catamenial rectal tenesmus – 22.11%, catamenial defecation disorders – 20.00%, catamenial intestinal subocclusion – 2.63%, catamenial meteorism – 4.21%, catamenial cough and hemoptysis – 1.05%, catamenial breast pain and spontaneous pneumothorax – 1.05%, Benjamin's symptom – 50.00%, weakness – 40.00%, catamenial bleeding from the scar – 2.63%, hiccups – 1.58%, frenicus symptom – 1.58% (Fig. 1).
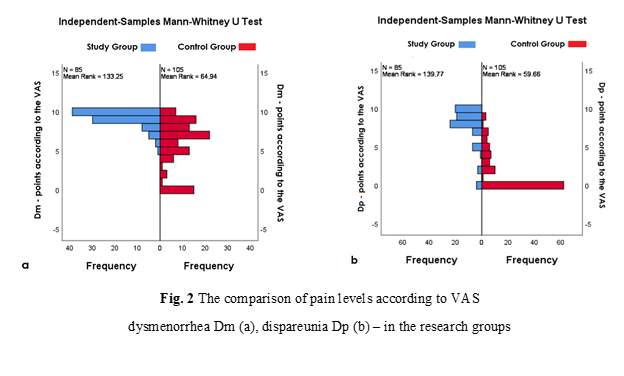
Pain syndrome. Pain levels on the VAS among women with DIE relative to the control group of patients were distributed as follows: the chronic pelvic pain – 7.90±0.21 points (95% CI; 7.47 – 8.34 points) vs the 2.41±0.45 points (95% CI; 1.49 – 3.33 points), the dysmenorrhea (Dm) – 9.02±0.17 points (95% CI; 8.67 – 9.38 points) vs the 5.31±0.52 points (95% CI; 4.24 – 6.37 points) (Figure 2a), the dyspareunia (Dp) – 7.85±0.33 points (95% CI; 7.18 – 8.53 points) vs the 2.18±0.47 points (95% CI; 1.23 – 3.13 points) (Figure 2b), the dysuria – 1.46±0.47 points (95% CI; 0.51 – 2.42 points) vs the 0 points, the dyschezia – 3.83±0.56 points (95% CI; 2.70 – 4.96 points) vs the 0 points. Statistical comparison showed significant differences between the study groups for chronic pelvic pain (U = 706.00, p < 0.01), dysmenorrhea (U = 1254.00, p < 0.01), and dyspareunia (U = 699.50, p < 0.01), while dysuria and dyschezia were identified as pathognomonic symptoms of deep endometriosis.
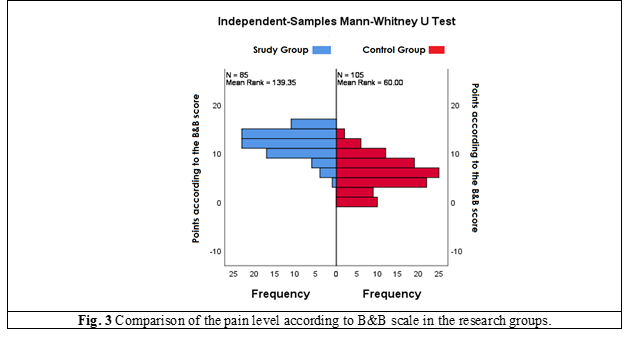
Objective pain assessment on the B&B score yielded the following results among DIE patients compared to the control group: the total symptom and sign severity score was 11.39±0.364 points (95% CI; 10.65 – 12.13 points) vs the 4.9±0.57 points (95% CI; 3.74 – 6.05 points) (Figure 3), the total pelvic pain score – 7.61±0.21 points (95% CI; 7.19 – 8.03 points) vs the 2.92±0.37 points (95% CI; 2.16 – 3.68 points), the total physical sign score – 3.78±0.22 points (95% CI; 3.34 – 4.22 points) vs the 1.98±0.26 points (95% CI; 1.46 – 2.49 points). Statistical comparison of pain intensity on the B&B scale between the study groups also revealed significant differences - U = 735.00, p < 0.01, confirming a more pronounced intensity of pain syndrome among women with deep infiltrating endometriosis.
Localization of deep endometriosis according to the #Enzian classification. Analysis of the localization of deep endometriosis foci in the main research group showed the following frequency distribution of this pathology by compartments of the #Enzian classification: localization in compartment A (the rectovaginal septum and vagina) – 55.3±5.3% (95% CI [45.2 – 65.5%]); in compartment B (the uterosacral ligament and pelvic walls) – 8.2±2.9% (95% CI [3.4 – 14.4%]); in compartment C (the sigmoid colon and rectum) – 1.2±1.2% (95% CI [0.0 – 3.8%]); in compartment FA (adenomyosis) – 17.6±4.2% (95% CI [10.0 – 26.3%]); in compartment FВ (the bladder) – 17.6±3.9% (95% CI [10.0 – 25.3%]); in compartment FI (the intestine) – 9.4±3.2% (95% CI [3.7 – 16.2%]); in compartment FO (other locations) – 12.9±3.7% (95% CI [6.0 – 20.5%]).
Correlation between #Enzian compartments and symptoms. Lesions of DIE in compartments A, B, FA, FI according to #Enzian classification statistically correlated with chronic pelvic pain, dysmenorrhea, dyspareunia and dyschezia > 7 points after VAS (p < 0.05). Additionally, lesions of DIE in compartment A according to #Enzian classification statistically correlated with catamenial rectal tenesmus, catamenial defecation disorders, Benjamin's symptom, and weakness (p < 0.05). At the same time, lesions of deep endometriosis in compartment B according to the #Enzian classification are statistically correlated with catamenial rectal tenesmus. The patient with a focus of deep endometriosis in compartment C according to the #Enzian classification presented with the following symptoms: chronic pelvic pain, dysmenorrhea, dyspareunia, and dyschezia > 7 points after VAS, catamenial rectal tenesmus, catamenial defecation disorders, Benjamin's symptom, and weakness. However, due to only one case, it would be inappropriate to discuss the statistical significance of these results. According to the #Enzian classification, lesions of endometriosis in compartment FA also show a statistically significant correlation with menometrorrhagia, catamenial rectal tenesmus, catamenial defecation disorders, Benjamin's symptom, and weakness (p < 0.05). Lesions of endometriosis in compartment FB, as per the #Enzian classification, demonstrate a statistically significant association with chronic pelvic pain, dysmenorrhea and dysuria > 7 points after VAS, catamenial hematuria, catamenial bladder tenesmus, hydronephrosis with ureteral stenting during pregnancy, Benjamin's symptom, and weakness (p < 0.05). Statistically significant correlations exist between lesions of endometriosis in compartment FI, categorized by the #Enzian classification, and catamenial defecation disorders, catamenial intestinal subocclusion, catamenial meteorism, Benjamin's symptom, weakness (p < 0.05). As per the #Enzian classification, lesions of endometriosis in compartment FO exhibit statistically significant correlations with chronic pelvic pain, dysmenorrhea, dyspareunia > 7 points after VAS, catamenial cough, hemoptysis, catamenial chest pain and spontaneous pneumothorax, catamenial hemorrhagic scar, hiccups, phrenic sign (p < 0.05).
Table 1 displays the frequency distribution of statistically significant symptoms depending on the localization of deep endometriosis, corresponding to the compartments of the #Enzian classification.
Table 1. Statistically verified correlation between #Enzian compartments and symptoms (p < 0.05). | ||||||
| A | B | FA | FB | FI | FO |
Chronic pelvic pain > 7 VAS | 85.1% | 100% | 100% | 86.7% | ||
Dysmenorrhea > 7 VAS | 97.8% | 100% | 100% | 93.3% | ||
Dyspareunia > 7 VAS | 93.6% | 100% | 86.7% | - | ||
Dysuria > 7 VAS | - | - | - | 86.7% | - | - |
Dyschesia > 7 VAS | 53.2% | 42.8% | 46.7% | - | - | |
Menometrorrhagia | - | - | 100% | - | - | - |
Hematuria | - | - | - | 60.0% | - | - |
Tenesmus vesical | - | - | - | 100% | - | - |
Hydronephrosis, ureteral stenting | - | - | - | 26.7% | - | - |
Tenesmus rectal | 57.4% | 71.4% | 73.3% | - | - | - |
Defecation disorders | 57.5% | - | 60.0% | - | 100% | - |
Intestinal suboccluzion | - | - | - | - | 62.5% | - |
Meteorism | - | - | - | - | 87.5% | - |
Cough, hemoptysis | - | - | - | - | - | 18.2% |
Chest pain | - | - | - | - | - | 18.2% |
Symptom Benjamin | 85.1% | - | 80.0% | 93.3% | 100% | - |
Weakness | 74.5% | - | 80.0% | 73.3% | 87.5% | - |
Hemorrhagic scar | - | - | - | - | - | 45.5% |
Hiccups | - | - | - | - | - | 27.3% |
- | - | - | - | - | 27.3% | |
Note: A – DIE in the rectovaginal septum and vagina; B – DIE in the uterosacral ligament and pelvic walls; FA - adenomyosis; FB – DIE in the bladder; FI – DIE in the intestine; FO – DIE in other locations | ||||||
Quality of life in endometriosis. According to the results of the EIQ questionnaire calculation, deep endometriosis significantly affected the majority of life determinants in patients compared to the control group of women, negatively influencing the quality of life. The physical health of the main research group's patients was reduced by 64.49±3.39% (95% CI; 57.63 – 71.34%), whereas in the control group it was reduced by 14.13±2.40% (95% CI; 9.26 – 18.99%). The main research group's patients experienced a 70.27±3.06% (95% CI; 64.08 – 76.46%) reduction in mental health compared to a 14.10±2.71% (95% CI; 8.61 – 19.60%) reduction in the control group. The social function of the main research group's patients was reduced by 52.68±4.38% (95% CI; 43.81 – 61.55%), whereas in the control group it was reduced by 4.72±1.82% (95% CI; 1.03 – 8.40%). In comparison to the control group, the patients in the main research group showed a 64.9±4.53% (95% CI; 55.74 – 74.07%) decrease in sexual function, while the control group experienced a 12.74±2.97% (95% CI; 6.76 – 18.73%) decrease. The fertility of patients in the main research group deteriorated by 90.93±3.54% (95% CI; 83.77 – 98.09%), in contrast to a 79.90±5.45% (95% CI; 68.86 – 90.93%) deterioration observed in the control group. The main research group's patients experienced a 58.76±4.58% (95% CI; 49.50 – 68.02%) reduction in work capacity compared to a 4.77±1.52% (95% CI; 1.68 – 7.86%) reduction in the control group. Relative to the control group, patients from the main research group exhibited a 44.27±4.12% (95% CI; 35.95 – 52.59%) decrease in educational attendance, whereas the control group demonstrated a reduction of only 3.64±1.33% (95% CI; 0.95 – 6.33%). The lifestyle of patients in the main research group deteriorated by 53.02±5.54% (95% CI; 41.83 – 64.22%) in contrast to a 2.28±1.24% (95% CI; -0.22 – 4.79%) deterioration observed in the control group. Comparison of EIQ questionnaire results revealed a statistically significant difference in the impact of pathologies in the research groups on physical health (U = 479.00, p < 0.01) and mental health of patients (U = 311.00, p < 0.01), on social function (U = 701.50, p < 0.01), sexual function (U = 690.50, p < 0.01), fertility (U = 1107.50, p = 0.001), work capacity (U = 841.00, p < 0.01), attendance of education (U = 567.50, p < 0.01), and lifestyle (U = 850.00, p < 0.01).
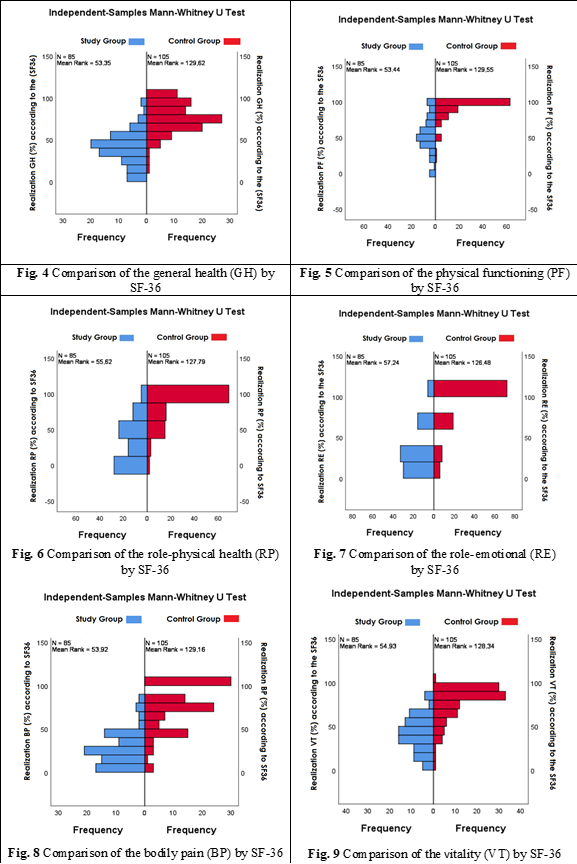
According to the SF-36 questionnaire calculation results, deep endometriosis significantly affected the quality of life of patients, as evidenced by the reduction in the realization of life determinants in women with deep endometriosis compared to similar data in the control group.
Thus, the realization of the general health potential (GH) among women in the main research group amounted to 42.15±3.42% (95% CI; 35.23 – 49.06%), compared to 76.23±3.02% (95% CI; 70.12 – 82.34%) in the research control group. The realization of the ability to perform usual physical activities (PF) among women in the main research group amounted to 58.54±4.11% (95% CI; 50.22 – 66.85%), compared to 92.18±1.95% (95% CI; 88.23 – 96.13%) in the research control group. The realization of performance of work or other duties (RP) among women in the main research group was 39.63±5.16% (95% CI; 29.20 – 50.07%), as opposed to 89.10±3.41% (95% CI; 82.20 – 96.01%) in the research control group. The performance of work or other duties in the context of emotional limitations, denoted as RE, among women in the main research group was measured at 37.78±4.54% (95% CI; 28.60 – 46.96%), contrasting with 85.59±3.99% (95% CI; 77.50 – 93.68%) in the research control group. The level of social activity and impact of physical or emotional health on social life (SF), among women in the main research group was measured at 47.12±1.02% (95% CI; 45.05 – 49.20%), contrasting with 51.41±1.01% (95% CI; 49.35 – 53.47%) in the research control group. Among women in the main research group, the degree of physical activity influenced by pain, referred to as BP, averaged 28.32±3.35% (95% CI; 21.53 – 35.11%), in contrast to 77.46±4.34% (95% CI; 68.67 – 86.25%) in the research control group. The energy and vitality, designated as VT, among women in the primary research cohort, averaged 42.44±2.80% (95% CI; 36.78 – 48.10%), compared to 76.26±3.30% (95% CI; 69.56 – 82.95%) in the control cohort. Women in the main research group demonstrated mental well-being and mental health, termed as MH, of 47.32±2.37% (95% CI; 42.51 – 52.12%), while those in the control group exhibited a level of 77.23±2.96% (95% CI; 71.23 – 83.23%) for mental well-being. The statistically significant difference in the research groups was confirmed in GH (U = 8045.50, p < 0.01, Figure 4), PF (U = 8037.50, p < 0.01, Figure 5), RP (U = 7852.50, p < 0.01, Figure 6), RE (U = 7715.00, p < 0.01, Figure 7), BP (U = 7997.000, p < 0.01, Figure 8), VT (U = 7911.00, p < 0.01, Figure 9), MH (U = 7991.50, p < 0.01, Figure 10). And only for the level of SF, a statistically significant difference was not determined (U = 4689.00, p = 0.473, Figure 11).
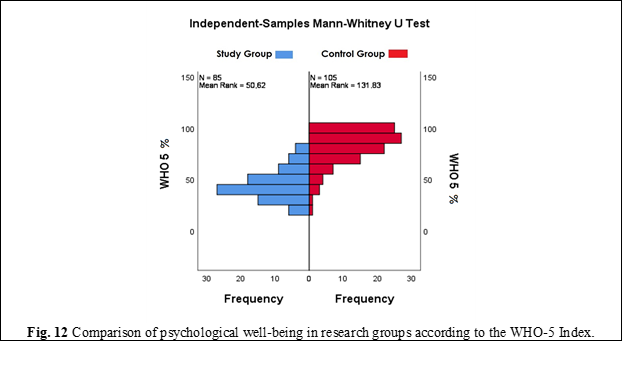
According to the WHO-5 Well-Being Index questionnaire, the level of psychological well-being in patients with deep endometriosis compared to the control group of women was 44.29±2.05% (95% CI; 40.15 – 48.44%) vs. 81.38±2.72% (95% CI; 75.88 – 86.89%). Despite the fact that the average level of psychological well-being did not reach 100% for all women included in this study, in the group of patients with deep infiltrating endometriosis, this parameter was significantly lower, as confirmed by a statistically significant difference in psychological well-being in the research groups - U = 8277.00, p < 0.01 (Figure 12).
Discussion
The results of our study offer valuable insights into the clinical and psychological characteristics of women with different phenotypes of endometriosis. Based on the data obtained, several key aspects can be highlighted.
Firstly, women suffering from deep infiltrating endometriosis (DIE) not only experience more intense pain, but also a wider spectrum of symptoms compared to patients with other phenotypes of this condition. This finding underscores the importance of early detection and accurate diagnosis of DIE to ensure timely treatment and improve the quality of life of patients. Secondly, our data indicate a high risk of recurrent surgical intervention in women with DIE, as this condition often may masquerade as extragenital pathologies. This highlights the importance of conducting more comprehensive clinical and paraclinical examinations when suspecting DIE to distinguish it from other nosological entities. Thirdly, our results confirm that severe pelvic pain syndrome is a characteristic feature of DIE and can be considered an important diagnostic indicator of this pathology. This emphasizes the need for an integrated approach to assessing pelvic pain syndrome in women suspected of having DIE. Fourthly, the results of our study, consistent with data from some international colleagues, have identified an association between the localization of DIE according to the #Enzian classification and clinical symptoms, which is an important aspect in the preoperative diagnostic process. These findings, together with existing literature, support recommending the #Enzian classification as the primary method for staging and mapping foci of DIE for surgical treatment by a multidisciplinary team. Finally, our results allow us to better understand the impact of DIE on the quality of life of patients. Patients with this type of endometriosis have a lower level of quality of life and psychological well-being compared to other phenotypes of endometriosis. This underscores the need for developing individualized treatment and support approaches for patients with DIE.
Overall, our results have important clinical and practical implications and can serve as a basis for further research and improvement of approaches to the diagnosis, treatment, and support of patients with DIE.
However, it is important to acknowledge the limitations of our study. Firstly, despite being prospective, our sample size was relatively small, which may limit the generalizability of our findings. Secondly, the prospective nature of the study does not preclude the possibility of selection bias and reliance on medical records for data collection. Future studies with larger sample sizes and more comprehensive data collection methods are needed to further validate our results and address these limitations.
Conclusions
High-intensity pain syndrome and extragenital symptoms correlated with compartments of #Enzian will assist in the preoperative multidisciplinary diagnosis of DIE. The high influence on life determinants, the low realization of life potential, and the low psychological well-being confirm the significant impact of DIE on QoL, suggesting its classification as a disability.
Competing interests
None declared.
Authors’ contributions
EI conceived the study and participated in the study design, contacted and included subjects in research, analyzed and calculated information from questionnaires, performed the statistical analysis of collected data, drafted the manuscript, reviewed the work critically, and approved the final version of the manuscript. NC proposed the study's area, conceived the study design, controlled main points of its realization, and reviewed the work critically. Both authors have read and approved the final version of the manuscript.
Acknowledgements and funding
We express our gratitude to Gheorghe Paladi, a professor and academician, who advised us to pursue research in the gynecological field and supported the realization of this study. The study had no external funding.
Patient consent
Obtained.
Ethics approval
The study protocol was approved by the Research Ethics Committee of Nicolae Testemiţanu State University of Medicine and Pharmacy (minutes nr. 38, from 21.05.2021).
Authors’ ORCID IDs
Elena Ivanova – https://orcid.org/0000-0001-8460-9849
Nadejda Codreanu – https://orcid.org/0009-0008-8740-0379
References
Donnez O. Conservative management of rectovaginal deep endometriosis: shaving should be considered as the primary surgical approach in a high majority of cases. J Clin Med. 2021 Nov 5;10(21):5183. doi: 10.3390/jcm10215183.
Imperiale L, Nisolle M, Noël JC, Fastrez M. Three types of endometriosis: pathogenesis, diagnosis and treatment. State of the art. J Clin Med. 2023 Jan 28;12(3):994. doi: 10.3390/jcm12030994.
Wild M, Miskry T, Al-Kufaishi A, Rose G, Crofton M. Medical management of deeply infiltrating endometriosis - 7 year experience in a tertiary endometriosis centre in London. Gynecol Surg. 2019 Dec 1;16(1):1-7. https://doi.org/10.1186/s10397-019-1065-9.
Keckstein J, Becker CM, Canis M, Feki A, Grimbizis GF, Hummelshoj L, et al. Recommendations for the surgical treatment of endometriosis. Part 2: deep endometriosis. Hum Reprod Open. 2020 Feb 11;2020(1):1-25. doi: 10.1093/hropen/hoaa002.
Bazot M, Bharwani N, Huchon C, Kinkel K, Cunha TM, Guerra A, et al. European society of urogenital radiology (ESUR) guidelines: MR imaging of pelvic endometriosis. Eur Radiol. 2017 Dec 5;27(7):2765-2775. doi: 10.1007/s00330-016-4673-z.
Johnson NP, Hummelshoj L, Adamson GD, Keckstein J, Taylor HS, Abrao MS, et al. World endometriosis society consensus on the classification of endometriosis. Hum Reprod. 2017 Feb 1;32(2):315-24. doi: 10.1093/humrep/dew293.
Vermeulen N, Abrao MS, Einarsson JI, Horne AW, Johnson NP, Lee TTM, et al. Endometriosis classification, staging and reporting systems: a review on the road to a universally accepted endometriosis classification. J Minim Invasive Gynecol. 2021 Nov 1;28(11):1822-48. doi: 10.1016/j.jmig.2021.07.023.
Keckstein J, Hudelist G. Classification of deep endometriosis (DE) including bowel endometriosis: From r-ASRM to #Enzian-classification. Best Pract Res Clin Obstet Gynaecol. 2021 Mar 1;71:27-37. doi: 10.1016/j.bpobgyn.2020.11.004.
Hernández Cardona MI, Ajewole C, Lewis H, Carrillo JF, Castellanos ME, Barish S, et al. Time to move beyond surgical classification systems for endometriosis. Int J Gynecol Obstet. 2023 Oct 1;163(1):58-62. doi: 10.1002/ijgo.14786.
Andres M de P, Lopes LA, Baracat EC, Podgaec S. Dienogest in the treatment of endometriosis: systematic review. Arch Gynecol Obstet. 2015 Sep 10;292(3):523-9. doi: 10.1007/s00404-015-3681-6.
Culley L, Law C, Hudson N, Denny E, Mitchell H, Baumgarten M, et al. The social and psychological impact of endometriosis on women’s lives: a critical narrative review. Hum Reprod Update. 2013 Nov;19(6):625-39. doi: 10.1093/humupd/dmt027.
De Graaff AA, D’hooghe TM, Dunselman GAJ, Dirksen CD, Hummelshoj L, Simoens S, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod. 2013 Oct;28(10):2677-85. doi: 10.1093/humrep/det284.
Oral E, Aydin O, Kumbak BA, İlvan S, Yilmaz H, Tustas E, et al. Concomitant endometriosis in malignant and borderline ovarian tumours. J Obstet Gynaecol. 2018 Nov 17;38(8):1104-9. doi: 10.1080/01443615.2018.1441815.
Tirlapur SA, Kuhrt K, Chaliha C, Ball E, Meads C, Khan KS. The “evil twin syndrome” in chronic pelvic pain: a systematic review of prevalence studies of bladder pain syndrome and endometriosis. Int J Surg. 2013;11(3):233-7. doi: 10.1016/j.ijsu.2013.02.003.
Yosef A, Allaire C, Williams C, Ahmed AG, Al-Hussaini T, Abdellah MS, et al. Multifactorial contributors to the severity of chronic pelvic pain in women. Am J Obstet Gynecol. 2016 Dec 1;215(6):760.e1-760.e14. doi: 10.1016/j.ajog.2016.07.023.
Adamson GD, Pasta DJ. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril. 2010 Oct;94(5):1609-15. doi: 10.1016/j.fertnstert.2009.09.035.
Ivanova E. Știința actuală în endometrioza profundă [Current science in deep endometriosis]. Bul Perinatol. 2022;1(93):5-15. Romanian.
Bień A, Rzońca E, Zarajczyk M, Wilkosz K, Wdowiak A, Iwanowicz-Palus G. Quality of life in women with endometriosis: a cross-sectional survey. Qual Life Res. 2020 Oct 1;29(10):2669-77. doi: 10.1007/s11136-020-02515-4.
Yela DA, Quagliato IDP, Benetti-Pinto CL. Quality of life in women with deep endometriosis: a cross-sectional study. Rev Bras Ginecol Obstet. 2020 Feb 1;42(2):90-5. doi: 10.1055/s-0040-1708091.