Introduction
Nitric oxide (NO) is a gaseous molecule that is a biological mediator that carries out important regulation of physiological processes necessary for the functioning of tissues [1-4]. NO participates in the activity of various organs and systems, contributing to both physiological and pathophysiological processes of the pancreas [3, 5, 6].
NO is produced by a family of enzymes called NO synthases (NOs), which convert L-arginine into NO and citrulline [1, 6]. The physiological effect of NO is caused by cyclic guanosine monophosphate (cGMP) and varies from modulation of the vascular system to regulation of immune processes and control of neuronal functions [7, 8]. NO plays an active role in pancreatic secretion, in the control of systemic blood pressure and active expansion of blood vessels, and is additionally involved in platelet aggregation, leukocyte activation, and their adhesion [1-3, 6].
The negative effect of NO content begins to manifest when its total concentration either sharply decreases or increases, which leads to functional and structural damage to the organ [4, 6, 9].
In conditions of pronounced oxidative stress, the physiological action of NO can be limited by the action of reactive oxygen species [1]. NO disintegrates into active forms of nitric oxide and, in combination with active forms of oxygen, can damage cells and thereby cause disease [1, 10]. Oxidative stress is one of the reasons for the development and progression of fibrosis of the gland parenchyma in chronic pancreatitis.
There is no doubt that NO is involved in the development of the pathological process. The participation of NO in the development of experimental pancreatitis is known, which is caused by the action of high doses of L-arginine (NO donor) on the pancreas [11, 12].
We hypothesized that nonspecific inhibition of all isoforms of NOs would also cause pancreatic damage and created a model of experimental pancreatitis based on NOs inhibition [4, 13]. Taking into account the fact that, along with the study of the mechanisms of development of chronic pancreatitis, the search for drugs to treat and inhibit the progression of this disease continues, we conducted an experimental research to study the therapeutic effect of arginine glutamate (the drug "Glutargin", "Zdorovya", Ukraine) in case of damage pancreas.
The drug glutargin is a salt of arginine and glutamic acid, along with a pronounced hypoammonemic effect; it has an antioxidant and antihypoxic effect. The analysis of the results obtained in the experiment when studying various aspects of the effect of glutargin on hepatocytes showed a number of positive effects of the drug: improvement of energy metabolism due to the primary accumulation of cellular energy in the form of creatine phosphate; correction of the acid-base state due to the normalization of the alkaline reserve of the blood; antioxidant and membrane-stabilizing effect due to the ability to reduce the level of lipid peroxidation products and increase the protective function of the endogenous antioxidant system, as well as stabilize hepatocyte membranes by reducing the activity of cytolytic enzymes (alanine and aspartate aminotransferases); anti-ischemic effect due to the optimization of oxygen transport and its consumption in tissues and increasing the body's resistance to hypoxia [14].
Besides, arginine in the composition of glutargin can serve as a donor of NO and thus ensure its physiological effect.
The purpose of the research is to study the protective effect of glutargin on the pancreas of rats with chronic pancreatitis induced by a NOs blocker.
Material and methods
Chronic pancreatitis was induced by intraperitoneal administration of the NOs blocker - N-nitro-L-arginine (L-NNA) (Sigma-Aldrich, USA) at a dose of 40 mg/kg of body weight. The solution was administered to animals intraperitoneally between 9:00 and 10:00 in the morning [4, 13]. Rats (n = 12) were intraperitoneally introduced with L-NNA at a dose of 40 mg/kg for 6 (n = 6) and 12 (n = 6) days. The control group (n = 12) consisted of rats that were intraperitoneally introduced with 0.9% NaCl solution. Rats were taken out of the experiment on days 6 and 12.
The effect of glutargin was studied on 21 laboratory white male Wistar rats weighing 180-230 g, which were randomly divided into control and two research groups. 16-20 hours before the experiment, animals were subjected to food deprivation with free access to water. Rats from group I (n = 7) were injected intraperitoneally with the NOs blocker L-NNA ("Sigma-Aldrich", USA) at a dose of 40 mg/kg for 12 days, rats from group II (n = 7) were injected intraperitoneally with glutargin 20 mg/kg, after 20 minutes L-NNA was administered intraperitoneally at a dose of 40 mg/kg for 12 days. The control group (n = 7) consisted of rats injected intraperitoneally with a 0.9% NaCl solution. The rats were removed from the experiment on the 45th day.
After the animals were removed from the experiment, blood was taken to determine the levels of protein-bound hydroxyproline (PBH) and free hydroxyproline (FH) [15], hexosamines (Ha) [16], malondialdehyde (MDA) [17], ceruloplasmin (CP) using a modified method Revin [16], nitrites/nitrates [18], α-amylase according to Karavey's method [19], lipase according to Loginov's method [20], trypsin - according to Erlanger in Shaternikov's modification [16].
Rat pancreas tissue was taken for histological examination. The pancreas was isolated and immediately fixed in Bowin's environment. Microscopic tissue sections with a thickness of 3-5 μm were stained with hematoxylin-eosin and according to Mallory-Slinchenko. Microscopy was performed at a magnification of X200-400.
To study the individual and typological features of the behavior of rats, which characterize individual resistance to emotional stress, testing was conducted in an open field [21].
Animals were removed from the experiment by administering a lethal dose of ketamine hydrochloride. Research was conducted in accordance with the main provisions of the European Convention on the protection of vertebrate animals used for research and other scientific purposes [22].
Descriptive and inductive statistics were used to analyze the obtained results. In the case of quantitative data and under the condition of their normal distribution, the mean and standard error of the mean were used. The Student's t-test was used to determine the reliability of differences. In the absence of a normal distribution, the median, minimum, maximum, upper and lower quartiles were used, and the significance of differences was determined by the Mann-Whitney U-test. To describe qualitative data, we used the frequency of detection of signs (%). In this case, the χ-test was used to determine the reliability of differences between groups. The indicator p < 0.05 was considered statistically significant. All calculations were performed in SPSS 9.0 for Windows (or Statistica 6) [23, 24]. The work was performed at the Institute of Gastroenterology of the National Academy of Medical Sciences of Ukraine.
The study was approved on September 10, 2008 by the Scientific Research Ethics Committee of the Institute of Gastroenterology of the National Academy of Medical Sciences of Ukraine (minutes No. 5).
Results
To achieve the goal, we used our previously developed model of chronic pancreatitis, which occurs as a result of long-term blocking of the nitroergic regulation by intraperitoneal injection of an NOs blocker [4, 13]. When simulating NOs deficiency on the 6th day, no deterioration in the general condition of the animals was found, and on the 12th day the behavior of the rats became more passive; appetite worsened, a slight decrease (by 5.0-9.0 grams) in the body weight of each animal was established.
A slight (p > 0.05) decrease in the level of nitrites/nitrates to 20.76±8.36 μmol/l was noted in the blood serum of rats on the 6th day compared to the control (32.61±4.55 μmol/l), and on the 12th day – a sharp increase to 80.22±19.91 μmol/l, (р < 0.05).
The inhibition of NOs on the 12th day was accompanied by a significant increase in the blood serum of rats of PBH content to 215.21±22.01 μmol/l compared to the control – 178.67±26.39 μmol/l, (p < 0.05), and FH up to 14.74±1.84 μmol/l, compared to the control – 9.96±0.71 μmol/l, (p < 0.05), which indicated enhanced synthesis and breakdown collagen.
On the 12th day, the content of Ha, which are inducers of fibrosis, also increased significantly (p < 0.001) to 5.90±0.25 g/l, control – 4.27±0.18 g/l, which indicated change in the state of connective tissue.
The introduction of the NOs blocker caused a violation of the excretory function of the pancreas, which was accompanied by phase changes of the enzymes of protein and carbohydrate metabolism. Thus, on the 6th day, the level of α-amylase increased to 311.26±37.39 mg/s·l, (p < 0.01) compared to the control – 96.02±20.30 mg/s·l, and on the 12th day it slightly decreased to 205.49±31.47 mg/s·l, but remained significantly higher than the control (p < 0.05). The level of trypsin on the 6th day increased to 10.45±1.76 μmol/ml·min (control – 4.19±0.92 μmol/ml·min), (p < 0.01), and on the 12th day decreased to 5.84±2.59 μmol/ml·min and did not differ from the control (p > 0.01).
There was also a violation of the incretory function of the pancreas, which was evidenced by a gradual increase in the level of glucose in the blood serum of rats, which on the 12th day was 4.20±0.22 mmol/l, (p < 0.05) compared to the control – 3.18±0.42 mmol/l.
The level of MDA on the 6th day increased to 4.94±0.35 nmol/ml in 1.4 times compared to the control – 3.62±0.13 nmol/ml, (р < 0.05), on 12th day was maximally elevated - (5.67±0.88) nmol/ml, 1.6 times compared to the control (р < 0.05).
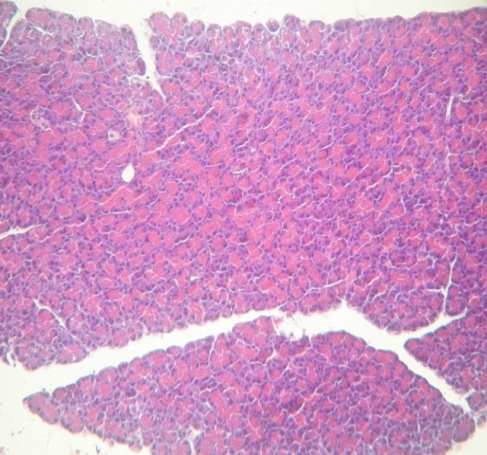
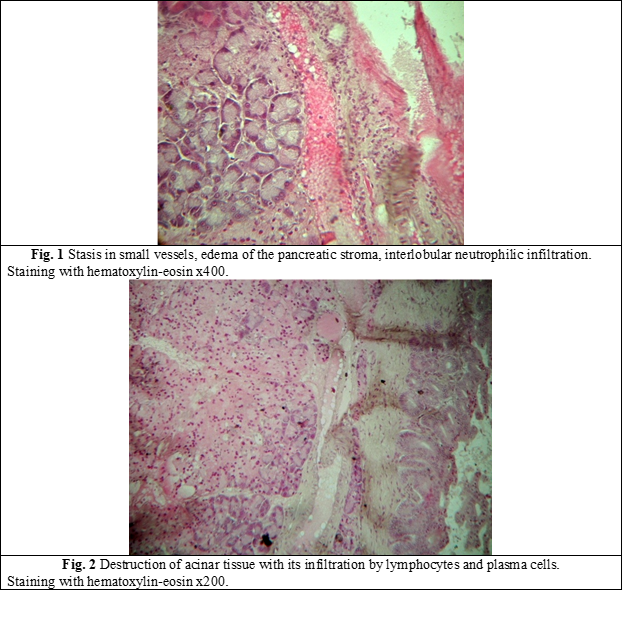
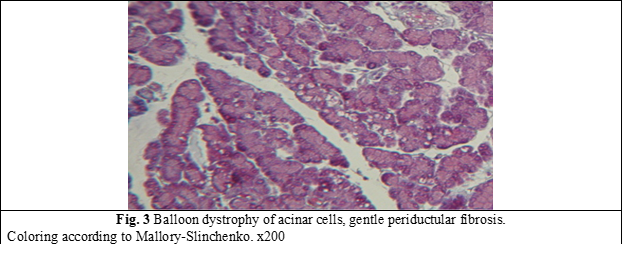
Morphological examination of the pancreas of rats revealed marked structural changes with stasis of formed blood elements in vessels, focal accumulation of leukocytes in the pancreatic parenchyma (Fig. 1). Dystrophy of acinar cells developed in some lobes (Fig. 2). In a number of cases, gentle fibrosis caused by the inflammatory process developed in the acinar tissue atrophy zone (Fig. 3).
The identified changes in total indicated that under the conditions of intraperitoneal administration of submaximal doses of a non-specific blocker of NOs, morphological changes are formed in the tissue of the pancreas of rats, which are characteristic of inflammation with chronicity of the pathological process, and the activation of the exocrine function of the pancreas is noted.
The second stage of our work was to study the effect of glutargin in pancreatitis induced by an NOs blocker.
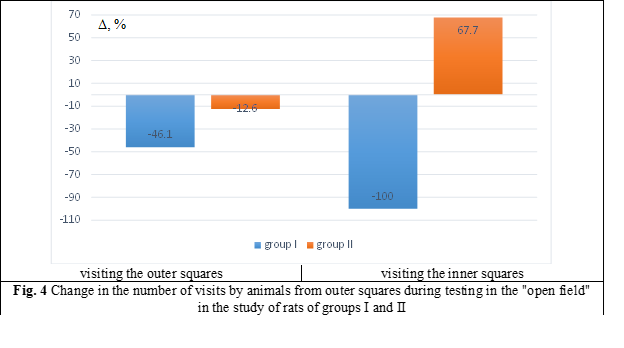
As a result of the conducted research, changes in the behavioral reactions of rats were established. Thus, in rats of the I group, after 45 days of the experiment (which included a 12-day administration of the NOs blocker), the total motor activity decreased in relation to the control values by 46.1% (p < 0.01). Whereas the rats of the II group, which additionally received glutargin, the indicators of general motor activity did not have significant differences in relation to the control values (Fig. 4).
Research activity in rats of the I group was significantly reduced, both by the indicators of racks by 67.5% (p < 0.001) and by the indicators of visiting the burrows by 48.9% (p < 0.01) in relation to the control values. In the II group, there was also a decrease in research activity, both by the rate of racks by 38.1% (p < 0.05) and by the rate of visits the burrows by 45.9% (p < 0.01) (Fig. 5).
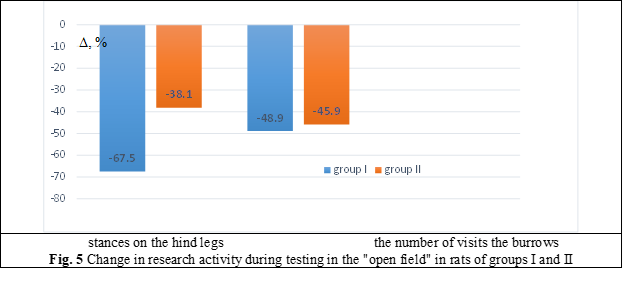
Table 1 presents the biochemical indicators of blood serum of rats of groups I and II in comparison with the control.
Table 1. Comparative characteristics of biochemical indicators of blood serum in rats (M±m) | |||
Parameters
| Control (n = 7) | І group L-NNA (n = 7) | ІІ group glutargin+L-NNA (n = 7) |
MDA, nmol/ml | 4.50±0.23 | 4.15±0.53 | 4.81±0.15 |
CP, mg/ml | 663.25±34.05 | 713.00±90.92 | 591.71±68.07 |
α-Amylase, mg/s·l | 56.82±1.87 | 58.66±1.74 | 57.52±2.47 |
trypsin, μmol/ml·min | 4.19±0.92 | 16.37±4.09* | 6.95±1.10 |
lipase, nmol/s·l | 0.87±0.086 | 0.79±0.09 | 1.26±0.07** |
PBH, μmol/l | 179.28±9.19 | 159.54±6.55 | 183.62±5.98 |
FH, μmol/l | 9.96±0.71 | 5.81±0.64* | 9.44±1.13 |
PBH/FH | 18.0±1.2 | 27.46±1.02 | 19.45±0.53 |
nitrites/nitrates, μmol/l | 32.61±1.63 | 36.46±3.87 | 33.59±5.84 |
Note: the Student's t-test was used; * - р < 0.05, ** - р < 0.01 - compared to the values of the control group; group I - L-NNA (N-nitro-L-arginine), group II – glutargin and L-NNA, MDA - malondialdehyde, CP - ceruloplasmin, PBH - protein-bound hydroxyproline, FH – free hydroxyproline | |||
In rats of the group I a high level of trypsin was determined, which was 4 times higher than the indicator of the control group (p < 0.01) and a significantly reduced level of FH. The ratio of PBH/FH in rats of the first group increased, which indicated the predominance of collagen synthesis processes over its degradation.
Most of the indicators in animals of group II were within the physiological norm, only the level of lipase was significantly increased (р < 0.01). In group II, the level of PBH and FH was not significantly different from the control. Therefore, according to these indicators; no collagen metabolism disorders were detected.
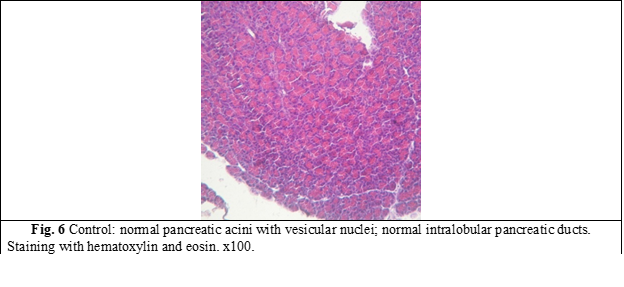
Structural changes of the pancreas were established during the morphological study of the pancreas of rats of the groups I and II. In fig. 6 the pancreas of a control group rat is presented.
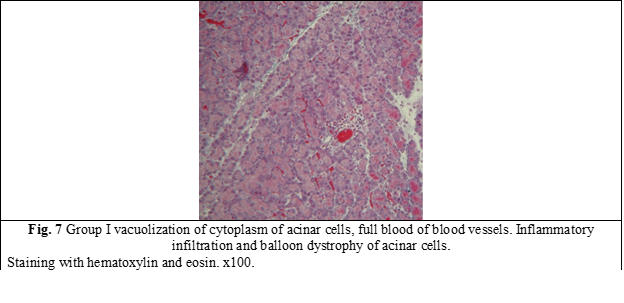
Rats of group I (which received only the NOs blocker) showed pronounced degenerative changes 45 days after the start of the experiment (Fig. 7). Dyscirculatory hypoxia with foci of dystrophy and atrophy of acinar tissue developed in the pancreas of rats (Fig. 7). In the majority of acinar cells, cytoplasmic vacuolization and a decrease in the number of zymogen granules were determined. The expansion of the interlobular ducts, expansion, and congestion of blood vessels, inflammatory infiltration, and balloon dystrophy of acinar cells were determined in the parenchyma of the pancreas.
Whereas in rats of group II (NOs blocker + glutargin) after 45 days, the morphological changes were much less pronounced (Fig. 8). Most of the acinar cells and lobules of the pancreas were almost normal and only a few acinar cells showed signs of dystrophy.
|
Fig. 8 Group II slightly expressed dystrophy of single acinar cells. Staining with hematoxylin and eosin. x100. |
Discussion
In this study, the model of chronic pancreatitis was induced by long-term intraperitoneal administration of the NOs blocker L-NNA, which caused the activation of lipid peroxidation (MDA), the increase in the concentration of toxic products and the activation of collagen synthesis (PBH), the violation of the excretory function of the pancreas. At the same time, morphological changes were formed in the tissue of the pancreas of rats, which are characteristic of inflammation with chronicity of the pathological process, fibrosis of the parenchyma of the gland in the zones of its atrophy.
The results obtained by us are consistent with the data of Werner J et al. (1998), who showed that NO donors reduced the severity of inflammation, pancreatic edema, intrapancreatic trypsinogen activation, and amylase secretion, while the NO blocker nitro-L-arginine methyl ester (L-NAME) increased the severity of inflammation and simultaneously reduced pancreatic tissue oxygenation gland [1, 6].
NOs blockers have also been reported to exacerbate cerulein-induced pancreatitis by modulating intrapancreatic secretion in vivo [1, 10, 25].
Other researchers have shown that NOs blockers can have a therapeutic effect in pancreatic disease. Camargo E. A. 2014 showed that NOS blockade reduces the severity of abdominal hyperalgesia and hyperamylasemia in pancreatitis induced by phospholipase A [26]. Demir I. E. et al. [27] proposed NOS inhibition as a new strategy for the treatment of unbearable pain in chronic pancreatitis. The authors found that mice suffering from the painful form of cerulein-induced pancreatitis could get significant relief when treated with a specific NOS inhibitor (Nω-propyl-L-arginine hydrochloride at a dose of 4 mg/kg).
Many scientific works show both positive and negative effects of NO induction in pancreatitis [1-3, 25, 27].
Such diversity of scientific results reflects the multifaceted nature of NO action, the variety of experimental approaches (including dosing of NO blockers/donors) and shows the perspective of further study on the role of NO in normal and pathological pancreas.
Our study showed that glutargin in chronic pancreatitis induced by a NOS blocker has a protective effect on the restoration of general behavioral reactions of rats, normalization of lipid peroxidation (MDA), antioxidant defense (CP) and collagen metabolism (PBH and FH) and prevents the development of pronounced structural changes in the pancreas.
Our data on the protective effect of glutargin coincides with the studies of V. I. Rusin et al. [28, 29]. The authors studied the effect of glutargin in patients with chronic pancreatitis and showed that the complex therapy of patients using glutargin contributed to the normalization of free amino acids in blood serum and was an effective tool for the correction of endothelial dysfunction.
In patients with peptic ulcer, the therapeutic effect of glutargin is realized through the limitation of oxidative stress, through the activation of glutathione peroxidase, superoxide dismutase, glutathione synthesis, which was proven in the experiment [30, 31].
Lebedeva T. (2008) found that glutargin prevents excessive activation of free radical oxidation processes and restores the activity of the antioxidant system in experimental adrenaline-induced acute myocardial damage [32].
Conclusions
To summarize our research, we can conclude that glutargin can be used in the complex treatment of patients with chronic pancreatitis as a drug that helps reduce the level of lipid peroxidation products, improves the state of the antioxidant defense system, helps normalize collagen metabolism, and prevents the development of pronounced structural changes in the pancreas glands.
Competing interests
None declared.
Authors’ contributions
OK – the idea and design of the study, participated in conducting the experiment, AR – participated in the development of the study design, performed experimental studies.
Acknowledgments
The author thanks Gaidar Y.A, MD. (Laboratory of pathomorphology of Institute of Gastroenterology of National Academy of Medical Sciences of Ukraine for participation in the research, assistance in obtaining data.
Ethics approval
The study protocol was approved by the Scientific Research Ethics Committee of the Institute of Gastroenterology of the National Academy of Medical Sciences of Ukraine (minutes No. 5 from September 10, 2008)
Authors’ ORCID IDs
Olena Krylova – https://orcid.org/0000-0002-3033-4912
Anatoliy Rudenko – https://orcid.org/0000-0001-9171-8875
References
Buchwalow I, Schnekenburger J, Samoilova V, Boecker W, Neumann J, Tiemann K. New insight into the role of nitric oxide pathways in pancreas. Acta Histochem Cytochem. 2018;51(6):167-172. doi: 10.1267/ahc.18028.
Stancill JS, Kasmani MY, Khatun A, Cui W, Corbett JA. Cytokine and nitric oxide-dependent gene regulation in islet endocrine and nonendocrine cells. Function (Oxf). 2021;3(1):zqab063. doi: 10.1093/function/zqab063.
Hegyi P, Rakonczay Z Jr. The role of nitric oxide in the physiology and pathophysiology of the exocrine pancreas. Antioxid. Redox Signal. 2011;15:2723-41. doi: 10.1089/ars.2011.4063.
Krylova OO. Rol’ NO v rozvytku khronichnoho pankreatytu [The role of NO in the development of chronic pancreatitis]. Buk Med Herald. 2011;15(2):218-221. Ukrainian.
Gheibi S, Ghasemi A. Insulin secretion: the nitric oxide controversy. EXCLI J. 2020;19:1227-45. doi: 10.17179/excli2020-2711.
Werner J, Fernández-del Castillo C, Rivera JA, Kollias N, Lewandrowski K B, Rattner DW, et al. On the protective mechanisms of nitric oxide in acute pancreatitis. Gut. 1998;43(3):401-407. https://doi.org/10.1136/gut.43.3.401.
Babushkina AV. L-arginin s tochki zreniia dokazatel’noi meditsiny [L-arginine from the point of view of evidence-based medicine]. Ukr Med Chasopys. 2010;(6/74):43-48. Russian. [cited 2022 Dec 12]. Available from: www.umj.com.ua/article/2937
Böger RH. The pharmacodynamics of L-arginine. J Nutr. 2007;137(6 Suppl 2):1650S-55S. doi: 10.1093/jn/137.6.1650S.
Rochette L, Lorin J, Zeller M, Guilland JC, Lorgis L, Cottin Y, Vergely C. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: possible therapeutic targets? Pharmacol Ther. 2013 Dec;140(3):239-57. doi: 10.1016/j.pharmthera.2013.07.004.
Vaquero E, Molero X, Puig-Diví V, Malagelada JR. Contrasting effects of circulating nitric oxide and nitrergic transmission on exocrine pancreatic secretion in rats. Gut. 1998 Nov;43(5):684-91. doi: 10.1136/gut.43.5.684.
Bohus E, Coen M, Keun HC, Ebbels TM, Beckonert O, Lindon JC, et al. Temporal metabonomic modeling of l-arginine-induced exocrine pancreatitis. J Proteome Res. 2008;7(10):4435-45. doi: 10.1021/pr800407j.
Fredstrom SB, Jessurun J, Gallaher DD. Pancreatitis induced in rats by repetitive administration of L-arginine. Pancreas. 2009 Apr;38(3):344-5. doi: 10.1097/MPA.0b013e318184ff83.
Krylova OO, Rudenko AI, Gaidar IuA. Method for modeling pancreatitis in experiment. Ukraine patent 61631 G 09B 23/00. 2011.
Babak OIa. Primenenie novogo otechestvennogo preparata glutargin v gastroenterologii [The use of a new domestic drug Glutargin in gastroenterology]. Suchasna Gastroenterologiia. 2003;2(12):85-88. Russian.
Osadchuk TK, Motin IuK, Osadchuk MA. Issledovanie oksiprolina v zheludochnom soke i ego diagnosticheskoe znachenie [Study of hydroxyproline in gastric juice and its diagnostic value]. Lab Delo. 1982;(4):16-18. Russian
Kamyshnikov VS. Spravochnik po kliniko-biohimicheskim issledovaniiam i laboratornoi diagnostike [Handbook of clinical and biochemical studies and laboratory diagnostics]. 3rd ed. Moscow: MEDpress-Inform; 2009. 896 p. Russian.
Ovsyannikova LM, Alekhina SM, Drobinskaya OV. [Biochemical and biophysical methods for assessing oxidative homeostasis disorders in persons exposed to radiation due to the Chernobyl accident: methodical recommendations]. Moscow; 1999. 18 p. Russian.
Metel'skaia VA, Gumanova NG. [Screening as a method for determining the serum level of nitric oxide metabolites]. Klin Lab Diagn. 2005;(6):15-18. Russian.
Caraway WT. A stable starch substrate for the determination of amylase in serum and other body fluids. Am J Clin Pathol. 1959;32(1):97-9. doi: 10.1093/ajcp/32.1_ts.97.
Loginov AS, Astashenkova KIu. Skrining-metod opredeleniia obshchei lipoliticheskoi aktivnosti krovi [Screening method for determining the total lipolytic activity of blood]. Lab Delo. 1986;8:463-466. Russian.
Buresh Ia, Bureshova O, Kh'iuston Dzh. Metodiki i osnovnye eksperimenty po izucheniiu mozga i povedeniia [Methods and basic experiments for studying the brain and behavior]. Moscow: Vysshaia shkola; 1991. 399 p. Russian.
Council of Europe. European Convention for the protection of vertebrate animals used for experimental and other scientific purposes, Strasbourg, 18.03.1986 [Internet]. Strasbourg: CE; 1986 [cited 2023 Nov 14]. Available from: https://rm.coe.int/168007a67b
Petri A, Sabin K, transl. Nagliadnaia statistika v meditsine [Medical statistics at a glance]. Moscow: Geotar-Med; 2003. 141 p. Russian.
Eniukov IS. [Methods, algorithms, programs for multidimensional statistical analysis]. Moscow: Finansy i statistica; 1986. 231 p. Russian.
DiMagno MJ, Williams JA, Hao Y, Ernst SA, Owyang C. Endothelial nitric oxide synthase is protective in the initiation of caerulein-induced acute pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2004;287(1):G80-7. doi: 10.1152/ajpgi.00525.2003.
Camargo EA, Santana DG, Silva CI, Teixeira SA, Toyama MH, Cotrim C, et al. Inhibition of inducible nitric oxide synthase-derived nitric oxide as a therapeutical target for acute pancreatitis induced by secretory phospholipase A2. Eur J Pain. 2014;18(5):691-700. doi: 10.1002/j.1532-2149.2013.00414.x.
Demir IE, Heinrich T, Carty DG, Saricaoglu ÖC, Klauss S, Teller S, et al. Targeting nNOS ameliorates the severe neuropathic pain due to chronic pancreatitis. EBioMedicine. 2019;46:431-443. doi: 10.1016/j.ebiom.2019.07.055.
Rusyn VI, Sirchak YeS, Kurchak NIu, Moskal OM. Dynamika pokaznykiv dysfunktsii endoteliiu u khvorykh iz khronichnym pankreatytom pislia kholetsystektomii pid vplyvom preparatu «Hlutarhin» [Dynamics of indicators of endothelial dysfunction in patients with chronic pancreatitis after cholecystectomy under the influence of the drug "Glutargin"]. Suchasna Gastroenterologiia. 2015;4(84):61-65. Ukrainian.
Rusyn VI, Sirchak YeS, Kurchak NIu. Dynamika rezerviv vilnykh aminokyslot syrovatky krovi u khvorykh na khronichnyi pankreatyt na foni kompleksnoi terapii iz zastosuvanniam L-arhininu L-hlutamat [Dynamics of provision of free aminoacids of blood serum at patients with chronic pancreatitis a background of complex therapy using L-arginine L-glutamate]. Visnyk Problem Biolohii i Medytsyny. 2013;1(3):184-187. Ukrainian.
Ponomarenko LA, Lykholat OA, Ponomarenko OA, Rudenko AI, Liapchenko VV. Sposib korektsii porushen stanu hlutationovoi systemy u khvorykh na kyslotozalezhni zakhvoriuvannia [A method of correcting the state of the glutathione system in patients with acid-dependent diseases]. Ukraine Patent 35533 A61K 31/00. 2008;18. Ukrainian.
Ponomarenko LA. Stan systemy hlutationu pry patolohii hastroduodenalnoi zony pry kombinovanomu vplyvi fizychnykh ta farmakolohichnykh chynnykiv [The state of glutathione system in the gastroduodenal zone pathology under the combined influence of physical and pharmacological factors] [dissertation] [Internet]. Ternopil, Ukraine: I. Horbachevsky Ternopil State Medical University; 2019. Ukrainian. [cited 2023 Nov 14]. Available from: https://repository.tdmu.edu.ua//handle/123456789/17067
Lebedeva TA. [Vplyv poperednykiv ta blokatoriv syntezu oksydu azotu na metabolichni protsesy v ushkodzhenomu adrenalinom miokardi v eksperymenti [The influence of precursors and blokators of nitric oxide synthesis on metabolic processes in the case of the adrenaline injury of myocardium in an experiment] [dissertation] [Internet]. Ternopil, Ukraine: I. Horbachevsky Ternopil State Medical University; 2008. 156 p. Ukrainian. [cited 2023 Nov 14]. Available from: https://repository.tdmu.edu.ua//handle/123456789/17372