Introduction
Non-Hodgkin lymphoma (NHL) is a lymphoid tissue tumor that develops from B cell progenitors, mature B cells, T cell precursors, and mature T cells. Non-Hodgkin lymphoma is divided into subgroups, each with its own epidemiology, etiology, immunophenotypic, genetic, clinical characteristics, and response to therapy [1, 2]. With an expected 544,000 new cancer cases in 2020, NHL was classified as the fifth to ninth most common malignancy in most countries worldwide [3]. NHL incidence in the Republic of Moldova is estimated to be 4.1 cases per 100,000 inhabitants [4, 5]. Based on data from the GLOBOCAN 2020 database provided by the World Health Organization's Global Cancer Observatory (GCO), 313 new cases of Non-Hodgkin's Lymphoma (NHL) were diagnosed in the Republic of Moldova in 2020. This ranked NHL as the 15th most common type of cancer in the country. These cases accounted for 2.2% of the total cancer diagnoses in the Republic of Moldova in 2020. The 5-year prevalence rate of NHL in the Republic of Moldova was 21.07 cases per 100,000 population [6].
Accurate prediction of the outcome of patients dealing with Non-Hodgkin's Lymphoma (NHL) is of paramount importance, as it plays a pivotal role in guiding the decisions surrounding their treatment approaches. This not only contributes to the potential improvement of patient outcomes but also ensures a more informed and effective management of their overall well-being and health. Arbitrarily, we can categorize prognostic scores into three main groups:
(a) Classic Prognostic Scores (IPI [7]; R-IPI [8]; MIPI [9]; FLIPI [10]). These encompass a combination of clinical and laboratory parameters that are accessible and commonly utilized in clinical practice.
(b) Integrated Prognostic Scores (R/R IPI [11]; m7-FLIPI [12, 13]). These involve the fusion of classic prognostic scores with molecular biology data, thereby adding a new layer of insight.
(c) Molecular-based Prognostic Scores (LymForest-25 Model [14]; IAC-FL [15]). This category involves the incorporation of molecular data and the utilization of machine learning technologies to enhance the precision of prognostication.
Certainly, the latter prognostication scores undeniably showcase an elevated refinement and heightened sensitivity when juxtaposed with the traditional prognostic scores [16]. Nevertheless, a notable downside associated with these advanced systems is their tendency to impose substantial financial strain on healthcare systems, consequently restricting their widespread utilization.
The aim of this study was to evaluate the utility of conventional scores based on biological and nutritional data in predicting overall survival (OS) in patients with primary nodal NHL.
Materials and methods
This study included 78 adult patients with NHL diagnosed and treated in the Chisinau Oncological Institute, during the period 2017-2021.
Prior to data collection, written informed consent was obtained from each study participant. Ethical approval was obtained from the Research Ethics Committee of Nicolae Testemițanu State University of Medicine and Pharmacy (minutes №1 from 03.07.2020).
Clinical-biological characteristics of NHL were collected from medical records. Additionally for each patient, prognostic scores (PS) were calculated in accordance with the recommendations of the original references. PS that were calculated are: International Prognostic Index (IPI) [7]; The combined index of hemoglobin, albumin, lymphocyte, and platelet (HALP score) [17]; Platelet to Lymphocyte Ratio (PLR) [18]; Neutrophil to Lymphocyte Ratio (NLR) [19]; Albumin/Globulin ratio (AG) [20] and Charlson comorbidity index (CCI) [21].
Statistical analysis of the results was carried out by using the standard packages of statistical programs SPSS for Windows (version 26). To create the database, the Microsoft Excel 7 version 2312 spreadsheet editor was used. To describe the nature of the distribution of quantitative features, standard methods of variational statistics were used with the determination of the arithmetic mean value of the variable (M) and the mean quadratic standard deviation (SD).
The average values in the study were presented in the form M ± SD. ROC curve analysis was used to determine the cutoff values of the evaluated PS in predicting mortality. To assess patient survival, Kaplan-Meier’s life-table method of forming survival curves, was used. Differences were considered significant if p < 0.05.
Results
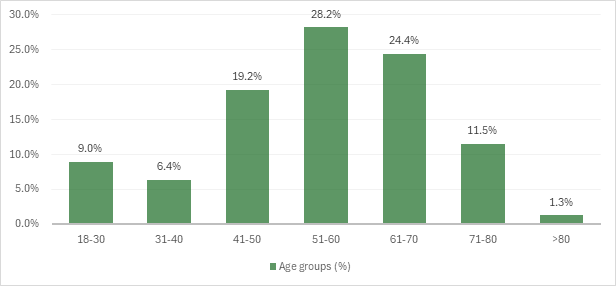
Out of the 78 patients participating in the study, 40 (51.2%) were women and 38 (48.8%) males. The mean age of the patients was 57.1 ± 10.2 years. The age categories most often affected by NHL in the study group were: age group 51-60 years – 22 (28.2%) patients, age group 61-70 years – 19 (24.4%) patients, followed by age group 41-50 years – 15 (19.2%) cases (fig. 1).
|
Fig. 1 Distribution of patients in the study group according to age categories. |
The analysis of the statistical distribution of the study group based on the primary area of the LN involvement showed that NHL onset occurred most frequently in peripheral lymph nodes (84.6 % of cases), followed by mediastinal lymph nodes (10.3 %), and abdominals lymph nodes (5.1%) (tab.1).
Table 1. Distribution of patients in the study group according to area of lymph nodes primary involved in the tumoral proliferation. | ||
Area of lymph nodes primarily involved | Number of patients | Frequency (%) |
Peripheral lymph nodes | 66 | 84.6 |
Mediastinal lymph nodes | 8 | 10.3 |
Intrabdominal lymph nodes | 4 | 5.1 |
Total | 78 | 100.0 |
The majority of patients in the study cohort developed aggressive forms of NHL, 57 (73.0%) patients, while indolent forms of NHL were identified in 21 (27.0%) cases. Among the aggressive forms of NHL, the most common histological subtype was diffuse large B-cell lymphoma, accounting for 46 (59.0%) of cases, followed by mantle cell lymphoma detected in 7 (9.0%) patients. NOS lymphomas and Burkitt lymphomas developed less frequently, in 3 (3.8%) and 1 (1.4%) cases, respectively. Among the indolent forms of NHL, small lymphocytic lymphoma was observed in 13 (16.7%) cases, followed by follicular lymphoma in 5 (6.4%) patients, and marginal zone lymphoma detected in 3 (3.8%) cases (fig. 2).
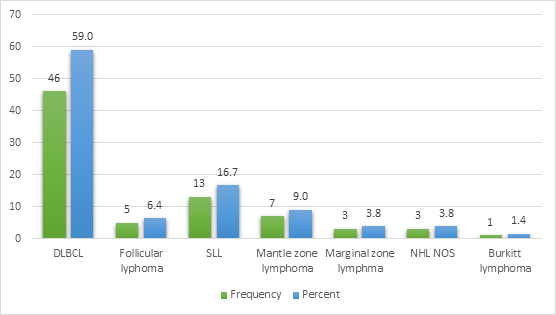 |
Fig. 2 Distribution of patients of the study group in regard to the type of Non-Hodgkin lymphoma. Note: DLBCL – diffuse large B cell lymphoma; SLL – small lymphocytic lymphoma; NHL NOS – Non-Hodgkin lymphoma not otherwise specified |
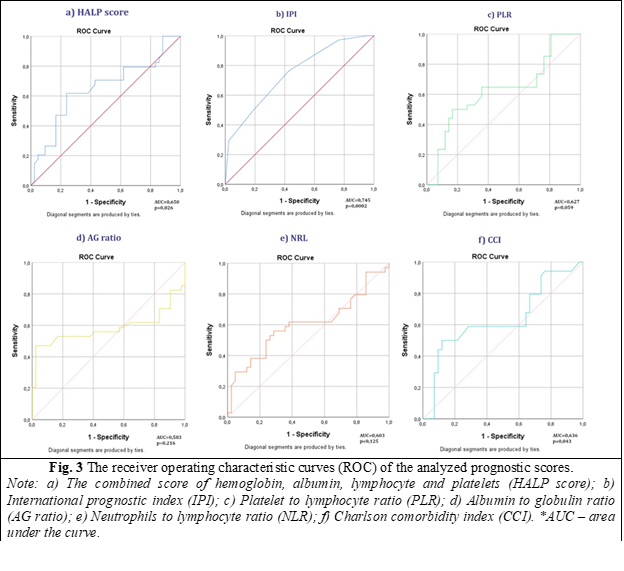
The subsequent phase involved assessing the performance potential of the analyzed prognostic scores (PS) to ascertain their suitability as effective predictors for NHL. To achieve this objective, Receiver Operating Characteristic (ROC) analysis was systematically applied to each of the scores. In the evaluation of prognostic scores (PS), it was observed that all scores exhibited an area under the curve (AUC) greater than 0.5, indicating a discernible discriminatory capacity.
However, not all scores demonstrated statistical significance. Specifically, the combined Hemoglobin Albumin Lymphocyte Platelets score (HALP) (AUC = 0.650; p = 0.026), International Prognostic Index (IPI) (AUC = 0,745; p = 0,0002), and Charlson Comorbidity Index (CCI) (AUC = 0.636; p = 0.043) were the only scores that yielded a p-value below the conventional significance threshold of 0.05 (fig. 3).
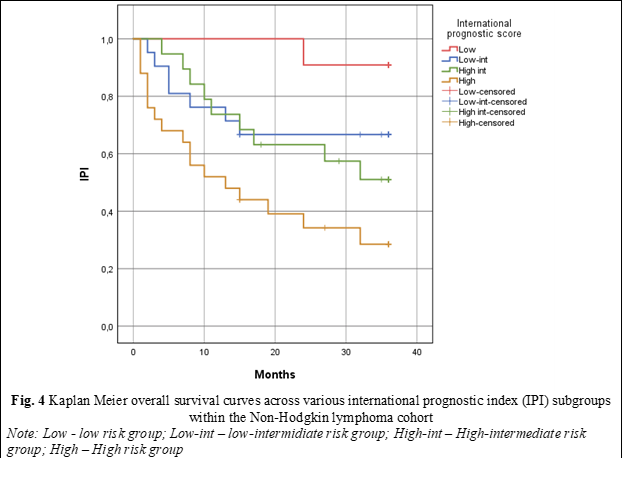
In order to validate the true prognostic impact of scores with prognostic significance (p < 0.005), Kaplan-Meier survival curves were constructed, with the use of HALP, IPI, and CCI scores serving as discriminant variables. In order to assess Overall Survival (OS) through the lens of the IPI score, patients were methodically stratified into 4 distinct prognostic subgroups based on the cumulative score: the low-risk subgroup (0-1 points); low-intermediate risk (2 points), high-intermediate risk (3 points), and high risk (4-5 points). Kaplan-Meier survival curve analysis unveiled that patients in the high-risk subgroup displayed a median OS of 13.0 ± 5.82, with a 95% Confidence Interval (CI) spanning from 1.57 to 24.42, and a statistically significant p-value of < 0.001. Conversely, for patients in the low-risk, low-intermediate risk, and high-intermediate risk subgroups, the median OS during the follow-up period was not reached (fig. 4).
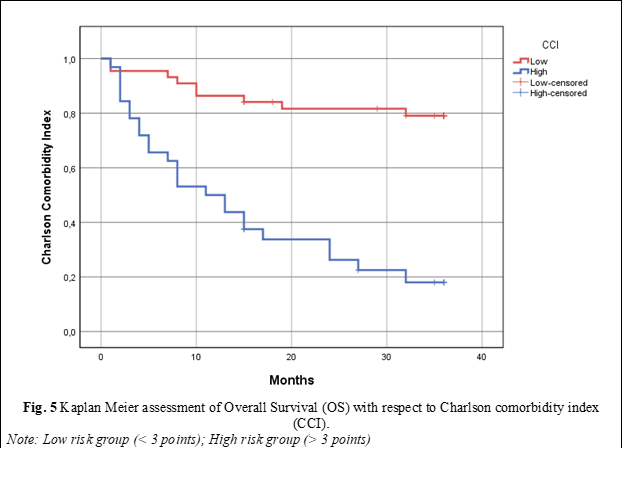
To assess the OS of patients within the study cohort utilizing the Charlson Comorbidity Index (CCI), patients were divided into two categories: those with a CCI < 3 were categorized into the low CCI index group, while those with a CCI score ≥ 3 points were placed into the high CCI index subgroup. Consequently, upon analyzing OS using the Kaplan-Meier survival curve, it was noted that patients with a high CCI score had a median OS of 11 ± 2.82 months, with a 95% Confidence Interval (CI) of 5.45-16.54, p < 0.0001. Patients within the low CCI score group demonstrated significantly superior OS rates, with the median overall survival not being reached within this cohort (fig. 5).
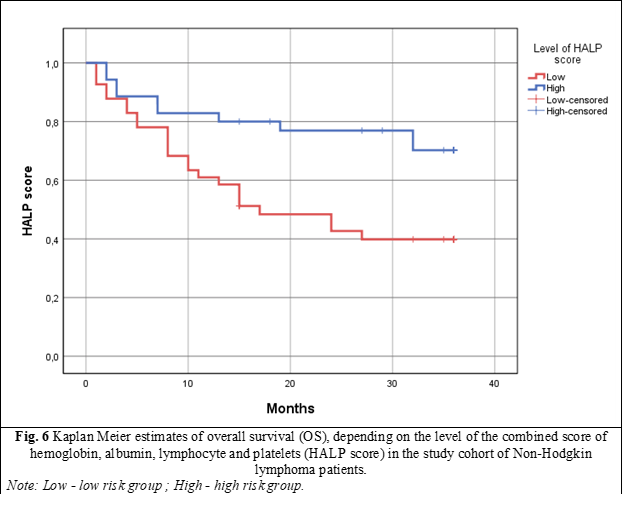
To evaluate the OS of patients within the study cohort using the HALP score, a cutoff value of 583.5 was initially determined based on ROC curve analysis. Subsequently, patients were divided into two distinct subgroups based on this threshold: those with a HALP score below 583.5, and those with a HALP score above 583.5. Evaluation of OS utilizing Kaplan-Meier survival curves revealed that patients in the low HALP score group had a median survival of only 17 ± 5.46 months, with a 95% confidence interval (CI) of 6.29-27.70, and a p-value of 0.009. In the high HALP score group, the median survival was not reached within the follow-up period (fig. 6).
Discussion
In 1863, Rudolf Virchow, upon visualizing leukocytes within neoplastic tissue, established the pioneering connection between inflammation and cancer [22]. Persistent inflammation and immune system activation have been identified nowadays as one of the pivotal factors in the pathogenesis of NHL [23].
Immunological markers and biomarkers of cancerogenesis are often relatively inexpensive to determine and easily interpretable. Although, at present, the medical scientific community cannot modify this pathogenetic chain in the evolution of cancer, what we can certainly do is utilize and benefit from the prognostic and sometimes diagnostic role of these markers. Several, scientific works have been undertaken to assess the utility of inflammatory and nutritional markers as plausible diagnostic and prognostic indicators across a spectrum of cancers, including gastric cancer [24, 25], hepatocellular carcinoma [26-28], prostate cancer [29, 30], gynecological cancers [31], etc.
Among lymphoproliferative neoplasms, the International Prognostic Index (IPI) is the most well-known scoring system, utilizing immunoinflammatory markers, among others, to predict overall survival in patients with aggressive NHL. The IPI score was published more than thirty years ago by Shipp et al. [7], and despite significant advancements in clinical classification for lymphomas, including immunohistochemical and molecular testing, it is still relevant today. This enduring relevance is evidenced by its continued use in most prospective, randomized trials to stratify risk and ensure balanced group allocation [32].
The HALP score, a composite index comprising routine blood tests data such as hemoglobin, albumin, lymphocyte, and platelet counts, serves as a comprehensive tool for evaluating various aspects of patients somatic status. Originally introduced as a predictive tool for gastric cancer prognosis [17], the HALP score has garnered attention for its potential utility in prognosticating outcomes across a wide spectrum of cancer types [33-36].
Recent studies have underscored the prognostic significance of the HALP score in hematological malignancies, notably multiple myeloma and aggressive NHL. In multiple myeloma, as it is showed in the work of Solmaz et al. [37] for instance, lower HALP scores have been associated with shorter overall survival, highlighting its potential as a prognostic marker in this context. Similarly, in aggressive NHL, such as diffuse large B cell lymphoma, lower HALP levels have been linked to adverse clinicopathological characteristics and diminished long-term survival rates, indicative of its prognostic relevance in these settings [38, 39]. The most important evidence on the aggressive NHL comes from a 2022 report published by Vlatka et al. [38], on 153 newly diagnosed diffuse large B-cell lymphoma. This study found that lower HALP was shown to be associated with unfavorable clinicopathological characteristics and a predictor of long-term survival. Patients with low HALP levels were also more likely to have B symptoms (p = 0.017), bone marrow infiltration (p = 0.001), and a poorer prognosis (p = 0.001). Moreover, 5-year survival was considerably lower for patients with Low HALP (47.3% vs. 79.5%, p = 0.001). In fact, on multivariable Cox regression, revealed that patients had a greater than 2.5 increased risk of death during the 5-year period if their HALP was low (p = 0.003). Our data tends to align with those presented by Vlatka et al. [38]. Thus, patients in our study cohort from the HALP scoring group had a median survival of 17 ± 5.46 months, with a 95% CI of 6.29-27.70, p = 0.009. In the high HALP score group, the median survival was not reached within the follow-up period.
In 1987, Charlson et al. [40] established the Charlson Comorbidity Index (CCI) to evaluate clinical comorbidities, which had been frequently used as a comprehensive assessment tool for patients with chronic diseases. The Charlson Comorbidity Index is a method of categorizing comorbidities of patients based on the International Classification of Diseases (ICD) diagnosis [41]. Each comorbidity category has an associated weight (from 1 to 6), based on the adjusted risk of mortality or resource use, and the sum of all the weights results in a single comorbidity score for a patient. A score of zero indicates that no comorbidities were found. The higher the score, the more likely the predicted outcome will result in mortality or higher resource use [40].
Reports of the clinical impacts of CCI in oncology have been made for several cancer types, including renal cell carcinoma [42] and non-small cell lung cancer [43]. Recent research on the impact of comorbidities on diffuse large B cell lymphoma patient outcomes has revealed that patients with high CCI have a lower rate of overall response, greater rates of toxicity due to medication, and a higher risk of fatal outcome [44-47]. For example, Eren et al. [47] demonstrated on a cohort of 170 with DLBCL that the CCI has an AUC of 0.628 (95% CI: 0.506–0.749), and patients with a CCI score of ≥ 4 had shorter OS compared to those with a score of < 4. Within our study cohort, the CCI showed an AUC of 0.636, and patients with a high CCI (>3 points) score had a median OS of 11 ± 2.82 months, with a 95% CI of 5.45-16.54, p < 0.0001. Patients in the low CCI score (<3 points) group demonstrated significantly superior OS rates, with the median overall survival not being reached within this cohort.
Conclusions
The prognosis of Non-Hodgkin Lymphoma depends on various factors. The use of conventional scores, which incorporate baseline nutritional and immunoinflammatory indicators, can significantly reduce the financial burden on healthcare systems in developing countries. Among the prognostic scores evaluated in this study, the International Prognostic Index (IPI), the Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) score, and the Charlson Comorbidity Index (CCI) have emerged as particularly sensitive predictors for estimating the overall survival (OS) of NHL patients.
Competing interests
None declared.
Acknowledgements and funding
The study had no external funding.
Patient consent
Obtained.
Ethics approval
The study was approved by the Research Ethics Committee of Nicolae Testemițanu State University of Medicine and Pharmacy (minutes №.1 from 03.07.2020).
Author’s ORCID ID
Victor Tomacinschii – https://orcid.org/0000-0002-5907-1714
References
Sapkota S, Shaikh H. Non-Hodgkin lymphoma. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan- [cited 2024 Feb 21]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559328/
Tomacinschii V, Robu M, Buruiana S, Finciuc V, Grecu A, Dudnic C, et al. Impact of targeted treatment in non-Hodgkin’s lymphoma with primary lymph node involvement. Mold Med J. 2021;64:56-61. https://doi.org/10.52418/moldovan-med-j.64-5.21.11.
Sedeta E, Ilerhunmwuwa N, Wasifuddin M, Uche I, Hakobyan N, Perry J, et al. Epidemiology of non-Hodgkin lymphoma: global patterns of incidence, mortality, and trends. Blood. 2022;140(Suppl 1):5234-5. https://doi.org/10.1182/BLOOD-2022-158830.
Robu M, Corcimaru I, Musteața L, Buruiană S, Tomacinschii V, Bogdanscaia N, Tuzlucov P; [Ministry of Health, Labor and Social Protection of the Republic of Moldova]. Limfoamele non-Hodgkin la adult: Protocol clinic naţional (PCN-64) [Non-Hodgkin's lymphomas in adults: National clinical protocol]. Chisinau: The Ministry; 2019. 36 p. Romanian.
Corcimaru I. Hematologie [Hematology]. Chisinau: Medicina; 2007. 388 p. Romanian.
World Health Organization, International Agency for Research on Cancer (IARC). Global Cancer Observatory. Republic of Moldova fact sheets. Lyon: IARC; 2021.
International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329(14):987-94. https://doi.org/10.1056/NEJM199309303291402.
Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109(5):1857-61. https://doi.org/10.1182/BLOOD-2006-08-038257.
Hoster E, Dreyling M, Klapper W, Gisselbrecht C, van Hoof A, Kluin-Nelemans HC, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111(2):558-65. https://doi.org/https://doi.org/10.1182/blood-2007-06-095331.
Solal-Céligny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, et al. Follicular lymphoma international prognostic index. Blood. 2004;104(5):1258-65. doi: 10.1182/blood-2003-12-4434.
Maurer MJ, Jakobsen LH, Mwangi R, Schmitz N, Farooq U, Flowers CR, et al. Relapsed/Refractory International Prognostic Index (R/R-IPI): an international prognostic calculator for relapsed/refractory diffuse large B-cell lymphoma. Am J Hematol. 2021;96(5):599-605. https://doi.org/10.1002/ajh.26149.
Lockmer S, Ren W, Ostenstad B, Brodtkorb M, Wahlin BE, Pan-Hammarstrom Q, et al. M7-FLIPI not valid in follicular lymphoma patients with first-line rituximab chemo-free therapy. Blood. 2018;132(Suppl 1):4154. https://doi.org/10.1182/BLOOD-2018-99-117949.
Pastore A, Jurinovic V, Kridel R, Hoster E, Staiger AM, Szczepanowski M, et al. Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: a retrospective analysis of a prospective clinical trial and validation in a population-based registry. Lancet Oncol. 2015;16(9):1111-22. https://doi.org/10.1016/S1470-2045(15)00169-2.
Mosquera Orgueira A, Diaz Arias JA, Cid Lopez M, Peleteiro Raindo A, Lopez Garcia A, Abal Garcia R, et al. Prognostic stratification of diffuse large B-cell lymphoma using clinico-genomic models: validation and improvement of the LymForest-25 model. Hemasphere. 2022;6(4):e706. https://doi.org/10.1097/HS9.0000000000000706.
Mosquera Orgueira A, Cid López M, Peleteiro Raíndo A, Abuín Blanco A, Díaz Arias JÁ, González Pérez MS, et al. Personally tailored survival prediction of patients with follicular lymphoma using machine learning transcriptome-based models. Front Oncol. 2022;11:705010. https://doi.org/10.3389/fonc.2021.705010.
Tomacinschii V, Mosquera Orgueira A, Santos CA, Robu M, Buruiana S, Fraga Rodriguez MF. The implication of next-generation sequencing in the diagnosis and clinical management of non-Hodgkin lymphomas. Front Oncol. 2023;13. https://doi.org/10.3389/FONC.2023.1275327.
Chen XL, Xue L, Wang W, Chen HN, Zhang WH, Liu K, et al. Prognostic significance of the combination of preoperative hemoglobin, albumin, lymphocyte and platelet in patients with gastric carcinoma: a retrospective cohort study. Oncotarget. 2015;6(38):41370-82. https://doi.org/10.18632/ONCOTARGET.5629.
Chen Y, Zhang Z, Fang Q, Jian H. Prognostic impact of platelet-to-lymphocyte ratio on diffuse large B-cell lymphoma: a meta-analysis. Cancer Cell Int. 2019;19:245. https://doi.org/10.1186/S12935-019-0962-3.
Wang S, Ma Y, Sun L, Shi Y, Jiang S, Yu K, et al. Prognostic significance of pretreatment neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in patients with diffuse large B-cell lymphoma. Biomed Res Int. 2018;2018:9651254. https://doi.org/10.1155/2018/9651254.
Bi XW, Wang L, Zhang WW, Yan SM, Sun P, Xia Y, et al. The pretreatment albumin to globulin ratio predicts survival in patients with natural killer/T-cell lymphoma. PeerJ. 2016;2016:e1742. https://doi.org/10.7717/PEERJ.1742.
Takeuchi J, Hojo A. Charlson Comorbidity Index is an independent prognostic factor among elderly patients with diffuse large B-cell lymphoma (DLBCL). Blood. 2009;114(22):3923. https://doi.org/10.1182/BLOOD.V114.22.3923.3923.
Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539-45. https://doi.org/10.1016/S0140-6736(00)04046-0.
Makgoeng SB, Bolanos RS, Jeon CY, Weiss RE, Arah OA, Breen EC, et al. Markers of immune activation and inflammation, and non-Hodgkin lymphoma: a meta-analysis of prospective studies. JNCI Cancer Spectr. 2018;2(4):pky082. https://doi.org/10.1093/JNCICS/PKY082.
Jaroenlapnopparat A, Bhatia K, Coban S. Inflammation and gastric cancer. Diseases. 2022;10(3):35. https://doi.org/10.3390/diseases10030035.
Zhang XY, Zhang PY, Aboul-Soud MA. From inflammation to gastric cancer: role of Helicobacter pylori. Oncol Lett. 2017;13(2):543-8. https://doi.org/10.3892/ol.2016.5506.
Bishayee A. The inflammation and liver cancer. Adv Exp Med Biol. 2014;816:401-35. https://doi.org/10.1007/978-3-0348-0837-8_16.
Yu LX, Ling Y, Wang HY. Role of nonresolving inflammation in hepatocellular carcinoma development and progression. NPJ Precis Oncol. 2018;2(1):6. https://doi.org/10.1038/s41698-018-0048-z.
Yang YM, Kim SY, Seki E. Inflammation and liver cancer: molecular mechanisms and therapeutic targets. Semin Liver Dis. 2019;39(1):26-42. https://doi.org/10.1055/S-0038-1676806.
de Bono JS, Guo C, Gurel B, De Marzo AM, Sfanos KS, Mani RS, et al. Prostate carcinogenesis: inflammatory storms. Nat Rev Cancer. 2020;20(8):455-69. https://doi.org/10.1038/s41568-020-0267-9.
Archer M, Dogra N, Kyprianou N. Inflammation as a driver of prostate cancer metastasis and therapeutic resistance. Cancers. 2020;12(10):2984. https://doi.org/10.3390/CANCERS12102984.
Goswami B, Rajappa M, Sharma M, Sharma A. Inflammation: its role and interplay in the development of cancer, with special focus on gynecological malignancies. Int J Gynecol Cancer. 2008;18(4):591-9. doi: 10.1111/j.1525-1438.2007.01089.x.
LaCasce AS. The International Prognostic Index: still relevant 30 years later. Haematologica 2023;108(6):1453-54. https://doi.org/10.3324/HAEMATOL.2023.283097.
Peng D, Zhang CJ, Tang Q, Zhang L, Yang KW, Yu XT, et al. Prognostic significance of the combination of preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients with renal cell carcinoma after nephrectomy. BMC Urol. 2018;18(1):20. https://doi.org/10.1186/S12894-018-0333-8.
Lou C, Jin F, Zhao Q, Qi H. Correlation of serum NLR, PLR and HALP with efficacy of neoadjuvant chemotherapy and prognosis of triple-negative breast cancer. Am J Transl Res. 2022;14(5):3240-46.
Toshida K, Itoh S, Kayashima H, Nagao Y, Yoshiya S, Tomino T, et al. The hemoglobin, albumin, lymphocyte, and platelet score is a prognostic factor for Child-Pugh A patients undergoing curative hepatic resection for single and small hepatocellular carcinoma. Hepatol Res. 2023;53(6):522-530. https://doi.org/10.1111/HEPR.13885.
Yalav O, Topal U, Unal AG, Eray IC. Prognostic significance of preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients undergoing curative resection for colorectal cancer. Ann Ital Chir. 2021;92:283-92.
Solmaz S, Uzun O, Sevindik OG, Demirkan F, Ozcan MA, Ozsan GH, et al. The effect of haemoglobin, albumin, lymphocyte and platelet score on the prognosis in patients with multiple myeloma. Int J Lab Hematol. 2023;45(1):13-9. https://doi.org/10.1111/IJLH.13958.
Vlatka P, Marko L, Stefan M, Dorian L. The hemoglobin, albumin, lymphocyte, and platelet (HALP) score is a novel prognostic factor for patients with diffuse large B-cell lymphoma. J Cancer Res Ther. 2022;18(3):725-732. doi: 10.4103/jcrt.jcrt_174_21.
Tomacinschii V, Buruiana S, Robu M. Clinical application of HALP score in the determination of nodal non-Hodgkin lymphoma prognosis. Documenta Haematologica: Rev Rom Hematol. 2023;1(2):51-8. https://doi.org/10.59854/dhrrh.2023.1.2.51.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40(5):373-83. https://doi.org/10.1016/0021-9681(87)90171-8.
Fritz AG, Percy C, Jack A, editors. International classification of diseases for oncology : ICD-O. 3rd ed. Geneva: WHO; 2000. 240 p.
Demircan NC, Alan Ö, Başoğlu Tüylü T, Akın Telli T, Arıkan R, Çiçek FC, et al. Impact of the Charlson Comorbidity Index on dose-limiting toxicity and survival in locally advanced and metastatic renal cell carcinoma patients treated with first-line sunitinib or pazopanib. J Oncol Pharm Pract. 2020;26(5):1147-1155. https://doi.org/10.1177/1078155219890032.
Gridelli C. Treatment of advanced non-small-cell lung cancer in the elderly: from best supportive care to the combination of platin-based chemotherapy and targeted therapies. J Clin Oncol. 2016;26(1):13-5. https://doi.org/10.1200/JCO.2007.14.1820.
Morrison VA, Hamilton L, Ogbonnaya A, Raju A, Hennenfent K, Galaznik A. Treatment approaches for older and oldest patients with diffuse large B-cell lymphoma – Use of non-R-CHOP alternative therapies and impact of comorbidities on treatment choices and outcome: a Humedica database retrospective cohort analysis, 2007-2015. J Geriatr Oncol. 2020;11(1):41-54. https://doi.org/10.1016/J.JGO.2019.07.025.
Lin TL, Kuo MC, Shih LY, Dunn P, Wang PN, Wu JH, et al. The impact of age, Charlson comorbidity index, and performance status on treatment of elderly patients with diffuse large B cell lymphoma. Ann Hematol 2012;91:1383–91. https://doi.org/10.1007/S00277-012-1463-9.
Jelicic J, Todorovic Balint M, Sretenovic D, et al. Enhanced International Prognostic Index (NCCN-IPI), Charlson Comorbidity Index and absolute lymphocyte count as predictors for survival of elderly patients with diffuse large B cell lymphoma treated by immunochemotherapy. Neoplasma. 2015;62(6):988-95. https://doi.org/10.4149/neo_2015_120.
Eren R, Serin I, Atak S, Pirdal BZ, Nizam N, Gemici A, et al. Charlson Comorbidity Index (CCI) in diffuse large B-cell lymphoma: a new approach in a multicenter study. Indian J Hematol Blood Transfus. 2023;39(2):191-9. https://doi.org/10.1007/S12288-022-01567-5.