Introduction
Worldwide, 844 million people suffer from chronic liver disease, with a mortality rate of two million deaths per year, including one million deaths from complications of cirrhosis. According to the National Bureau of Statistics in the Republic of Moldova, over 10000 patients with liver cirrhosis were registered in 2019, and 8962 in 2021 [1]. The main causative factors of liver cirrhosis are hepatitis B and C viruses, followed by non-viral causes, excessive alcohol consumption, and lipid dystrophy of the liver. As liver cirrhosis is often asymptomatic until decompensation occurs, many patients remain undiagnosed until complications arise. The main complications include ascites, spontaneous bacterial peritonitis (SBP), digestive bleeding from esophagogastric varices, and hepatic encephalopathy [2].
Bacterial infections in cirrhotic patients represent a major clinical problem, occurring 4-5 times more frequently compared to the general population. These infections can increase mortality by leading to acute-on-chronic liver failure, subsequent decompensation, and multiorgan failure. Sudden-onset spontaneous bacterial infections without an obvious source are specific to patients with decompensated liver cirrhosis and include spontaneous bacterial peritonitis, spontaneous bacteremia, and spontaneous bacterial empyema (infected hepatic hydrothorax). Despite advances in intensive care and prophylactic antibiotic usage, SBP remains the most common and significant bacterial infection in cirrhotic patients with ascites due to its considerable morbidity and mortality [3]. Spontaneous bacterial ascitic peritonitis occurs in 9% of hospitalized patients with liver cirrhosis and ascites and accounts for 25% of all infections in patients with liver cirrhosis [4]. Mortality due to untreated spontaneous bacterial peritonitis can reach up to 90% in patients with decompensated hepatic cirrhosis, but it decreases significantly, by up to 20%, with early diagnosis and prompt initiation of treatment [5].
In this article, the early initiation of treatment will be discussed using a patented procedure developed at the Scientific Research Laboratory of Hepatic Surgery, Nicolae Testemițanu State University of Medicine and Pharmacy for patients with cirrhosis.
The aim of the research is to determine the possibilities of laparoscopy with sanitation and drainage of the abdominal cavity, combined with postoperative peritoneal lavage with antibiotics, in the treatment of spontaneous ascitic peritonitis in patients with decompensated liver cirrhosis.
Materials and methods
We conducted a retrospective descriptive study on patients admitted with decompensated liver cirrhosis and ascites, and peritonitis between January 2012 and December 2021. Patients who underwent surgical drainage of the abdominal cavity by laparoscopy with postoperative peritoneal lavage with antibiotics were selected. The patients were treated at the Constantin Tibirna Surgery Department No.2, Holy Trinity Municipal Clinical Hospital, and the Scientific Research Laboratory of Hepatic Surgery, Nicolae Testemițanu State University of Medicine and Pharmacy. The data were extracted from the medical records of the hospital archive, and a patient database was compiled. Data analysis was performed using simple statistical calculations.
The study consists of analyzing the role of laparoscopic sanitation of the abdominal cavity in patients with liver cirrhosis and spontaneous bacterial ascites. The research was approved by the Research Ethics Committee of Nicolae Testemițanu University No. 15 from 28.02.2022 of the Republic of Moldova. The project was funded by the National Agency for Research and Development of the Republic of Moldova and conducted at a large tertiary medical center – the Holy Trinity Municipal Clinical Hospital. Patients with non-cirrhotic ascites were not considered for evaluation.
All patients underwent a diagnostic algorithm consisting of:
Chest radiography to evaluate pleuritis.
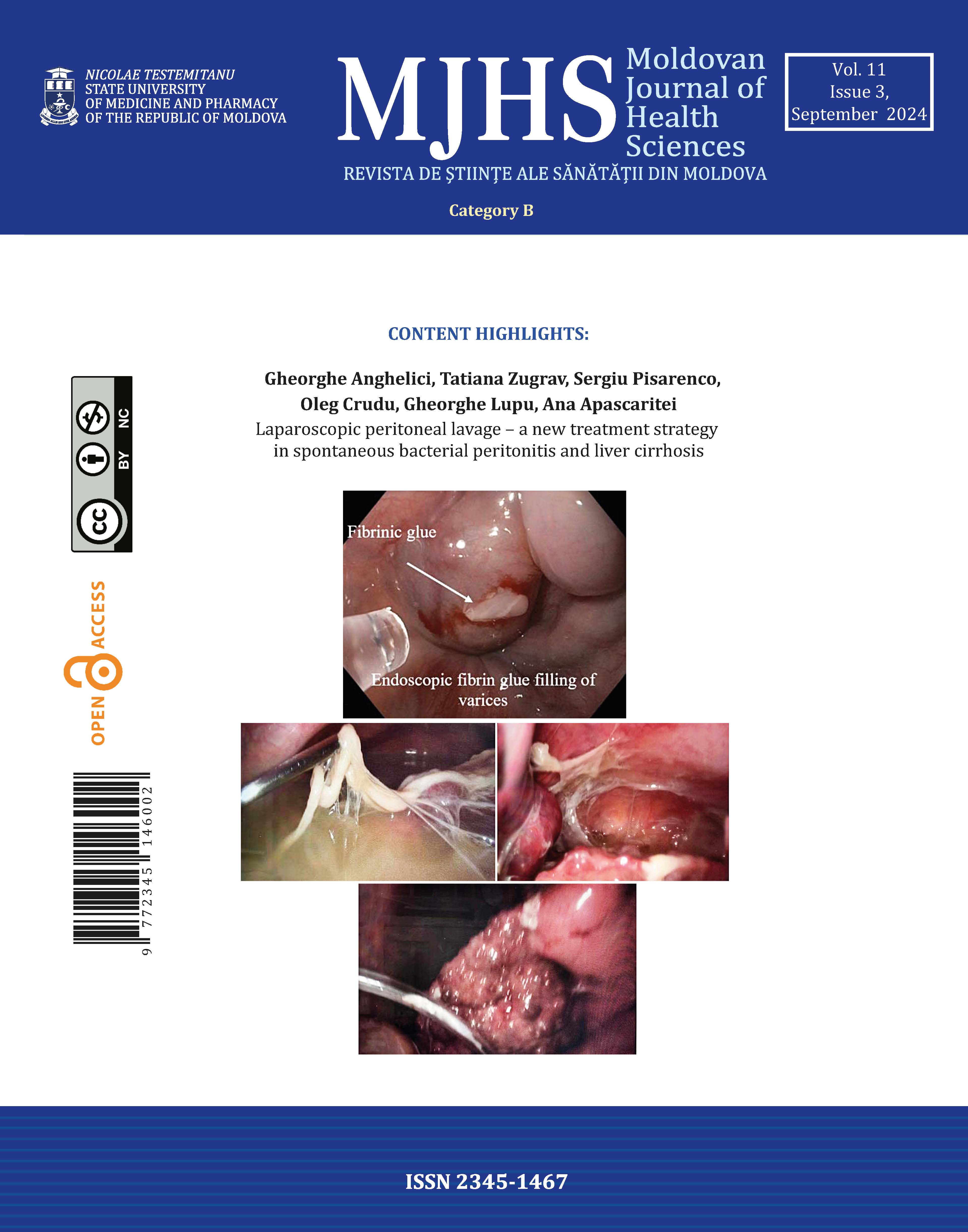
Esophagogastroduodenoscopy to prevent possible bleeding. Preoperative endoscopic fibrin glue filling of the esophageal and gastric varices was performed on 49 patients (60%) (Figure 1).
Laboratory analysis of ascitic fluid, including ascitic neutrophil count and ascitic fluid culture.
General analysis of urine and blood.
Ultrasonography of the abdomen and pelvis.
The diagnosis of SBP was based on criteria recommended by the International Ascites Club and published in 2000 [6].
Preoperative management
All patients received thorough preoperative preparation, including evaluation and correction of hepatic status, thoracocentesis, and dosed preoperative microlaparocentesis (Figure 2), with perfusion of albumin to avoid post-paracentesis circulatory dysfunction and to reduce acute hepatic failure.
Dosed microlaparocentesis consisted of abdominal cavity puncture on the abdominal flank with a 14G vein cannula under local anesthesia with 2% lidocaine solution (2 ml) to evacuate ascitic fluid through the perfusion drainage system in a dosed manner. This avoids circulatory dysfunction post-paracentesis. During the same session, albumin (6-8 g/l), hepatoprotective drugs, and prokinetic drugs were administered according to the national protocol for ascites in cirrhotic patients [7].
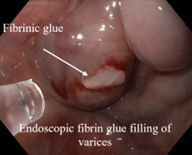 |
Fig. 1 Preoperative management of patients with decompensated liver cirrhosis and ascites complicated by spontaneous bacterial peritonitis – preoperative endoscopic fibrin glue filling of the esophageal and gastric varices. |
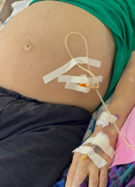 |
Fig. 2 Preoperative management of patients with decompensated liver cirrhosis and ascites complicated by spontaneous bacterial peritonitis – dosed microlaparocentesis with a 14G venous catheter. |
Minimally invasive surgical treatment of SPB
The minimally invasive surgical treatment of patients with cirrhosis and SPB includes two stages:
Laparoscopic complete evacuation of ascitic fluid with peritoneal lavage and drainage of the abdominal cavity.
Under general anesthesia, the first, optical, trocar was placed through the umbilical site. The working trocar, 5 mm in size, was placed on the right flank. Through the working trocar, the entire abdominal cavity was reviewed, and all the ascitic fluid from all spaces was evacuated, allowing for the inspection of abdominal organs (Figure 3).
The next step is to debride all fibrinous smudges and adhesions from the intraperitoneal cavity (Figure 3A) and the parietal peritoneum and diaphragm (Figure 3B). Then, lavage is carried out with 1.0–2.0 L of saline, which is completely aspirated. A mixture containing hyaluronidase (640-1080 CU) dissolved in 200-400 ml of saline and ceftriaxone (2-4 g) is then introduced simultaneously. Drainage tubes are placed in the Douglas pouch and right subdiaphragmatic space (Figure 3C).
Post-operative irrigation of the abdominal cavity
In the postoperative period, third-generation cephalosporins and ciprofloxacin, along with the hyaluronidase enzyme and 2-3 L of saline solution, were introduced daily through the drainage tubes and removed after several hours. This procedure, called post-operative irrigation of the abdominal cavity, was performed daily for 5 days.
Postoperative lavage of the peritoneal cavity removes residual fibrin and infections, decreases peritoneal edema, and improves its absorbent properties.
82 patients with decompensated liver cirrhosis and ascites for more than one month prior, and SBP indicated by an ascitic neutrophil count >250 cells/mm3 in the absence of an intra-abdominal and surgically treatable source of sepsis were analyzed. These patients underwent diagnostic-therapeutic laparoscopy with sanitation and drainage of the abdominal cavity, followed by postoperative lavage and antibiotics.
Inclusion criteria: decompensated liver cirrhosis with ascites for more than one month prior; SBP indicated by an ascitic neutrophil count >250 cells/mm3 or patients with positive bacteriological examination of ascitic fluid.
Exclusion criteria: absence of any other sources of infections, such as pneumonia, renal infections, surgical treated source of intrabdominal infections; encephalopathy; any states that serves as contraindication for pneumoperitoneum; patients with non-cirrhotic ascites; pleuritis; patients with pneumonia.
Results
Patients’ characteristics are shown in Table 1. The mean MELD (Model for End-Stage Liver Disease) score in this group was 21. Most of the patients had liver cirrhosis of viral etiology, primarily of HCV origin (Figure 4).
Table 1. Demographic and clinical data | |
No. | 82 |
Gender Male Female |
49 (60 %) 33 (40 %) |
Age | 53.9 (range, 28 – 71) |
Child-Pugh Class C | 82 (100%) |
MELD score | 21 (9-31) |
Serum bilirubin (mg/dL) | 40 (26-38) |
Serum albumin (g/dL) | 20 (14-24) |
Prothrombin time (%) | 58 (40-65) |
Serum creatinine (mg/dL) | 93 (70-120) |
Prophylactic endoscopic sealing of eso-gastric varices | 49 (60 %) |
Time of diagnosis establishment from admission to hospital | 72 h |
Time of onset of ascites | 15 months |
Recent evacuation laparocentesis, 1 month | 9 |
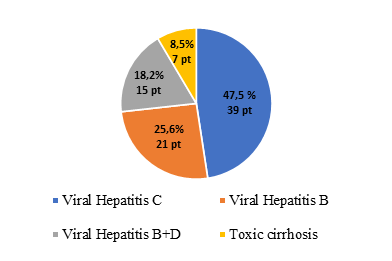 |
Fig. 4 Major etiological factors of liver cirrhosis. |
Following the analysis of the patient group, it was observed that patients with liver cirrhosis and decompensated ascites who were diagnosed with SBP through the analysis of ascitic fluid by diagnostic laparocentesis did not present a specific clinical picture, nor did they have significant complaints. A classic clinical picture that would clearly indicate a diagnosis of SBP was present in only 24 patients, specifically those with bacteriascites. Most patients exhibited general symptoms, such as general asthenia and intestinal motility disorders. Some patients had nonspecific abdominal symptoms, such as mild abdominal pain, constipation, or frequent semi-formed stools, and only 10 out of 82 patients with spontaneous bacterial peritonitis experienced abdominal pain with signs of peritonism (Table 2).
Table 2. Symptoms and signs of spontaneous bacterial peritonitis and its variants | |||
Symptoms and signs | SBP 82 pt (100%) | Bacteriascites 24 pt (29.2%) | CNNA 58 pt (70.7%) |
Abdominal pain | 14 (17 %) | 12 (85.7) | 2 (14.2) |
Abdominal tenderness | 46 (56 %) | 24 (52.1) | 22 (47.8) |
Rebound | 10 (19 %) | 7 (70%) | 3 (30%) |
Nausea | 34 (41.4%) | 20 (58.8) | 14 (41.1) |
Fatigue | 67 (81.7 %) | 24 (35.8) | 43 (64.1) |
Vomiting | 17 (20.7 %) | 9 (52.9) | 8 (47.0) |
Fever | 9 (10.9 %) | 7 (77.7) | 2 (22.2) |
Diarrhea | 21 (25.6 %) | 8 (38.0) | 13 (61.9) |
Constipation | 38 (46.3 %) | 30 (78.9) | 8 (21.0) |
Signs of dynamic ileus | 3 (3.6 %) | 3 | 0 |
Encephalopathy | 21 (25.6 %) | 18 (85.7) | 3 (14.2) |
Upper digestive bleeding | 17 (20.7 %) | 14 (82.3) | 3 (17.6) |
Note: SBP - Spontaneous Bacterial Peritonitis; CNNA – Culture-Negative Neutrocytic Ascites. | |||
The volume of ascitic fluid withdrawn per paracentesis ranged from 8 to 16 L. The ascitic protein content was 14 g/L, and the mean neutrophil count was 290 cells/mm3. Positive ascitic fluid bacterial culture was detected in 29.2% of patients (24 patients), while 70.7% (58 patients) had culture-negative neutrocytic spontaneous bacterial peritonitis. Ascitic fluid characteristics are shown in Table 3. The variants of SBP in our study population are shown in Figure 4.
Table 3. Ascitic fluid characteristics. | |
Average volume of ascitic fluid withdrawn per paracentesis (L) (range) | 11 (8-16) |
Neutrophil count/ mm3 (range) | 29 0 (180-350) |
Total cell count/ mm3 (range) | 370 (280-580) |
Protein content (g/L) | 14 (5-47) |
LDH, U/L | 63 (45-90) |
Note:LDH – Lactate Dehydrogenase. | |
|
Fig. 4 Positive ascitic fluid bacterial culture was detected in 29.2% of patients (24 patients); 70.7 % (58 patients) had culture-negative spontaneous bacterial peritonitis. |
Based on the bacteriological evaluation, the most frequent bacterial species was E. coli, found in 54.1% of patients (13 patients). No cases of anaerobic infection were registered.
Table 4. Bacterial species detected in ascitic fluid |
|
Microbial agents in ascitic fluid simple | No. of patients (%) |
Escherichia coli | 13 (54.1) |
Staphylococcus aureus | 4 (16.6) |
Streptococcus haemolyticus | 3 (12.5) |
Klebsiella pneumoniae | 2 (8.3) |
Staphylococcus epidermidis | 2 (8.3) |
In the early postoperative period, patients subjectively reported an improvement in their general condition and did not present complications related to the surgery. Mortality at 1 month was 8.5% (7 patients) due to progressive liver failure. Readmissions for SBP at 1 month were 6% (5 patients).
Discussion
The current theory of the pathogenesis of SBP in patients with cirrhosis and ascites suggests that repeated episodes of bacterial translocation from the intestinal lumen to the mesenteric lymph nodes lead to systemic bacterial inoculation, representing key steps in the development of SBP. However, most episodes of systemic bacteremia remain undetected [8].
Patients with SBP may present with intestinal ileus, fever, diffuse abdominal pain, and a palpable abdomen with diffuse, unclear peritoneal signs. However, up to one-third of patients with spontaneous peritoneal infections may be completely asymptomatic or present with only encephalopathy and/or acute renal failure [9, 10]. The diagnosis of SBP is established by performing laparocentesis on admission, which reveals an absolute neutrophil count in the ascitic fluid greater than 250/mm3 [11]. Ascites itself is not fatal unless it becomes infected (spontaneous bacterial peritonitis). The infection, in turn, often precipitates hepatorenal syndrome, increases the risk of bleeding from esophageal varices, and, if left untreated, can lead to death [12].
Intestinal permeability dysfunction in patients with decompensated liver cirrhosis is the primary event triggering spontaneous bacterial peritonitis. The passage of infection into the ascitic fluid initiates an inflammatory process, manifested by an increase in the number of neutrophils [2, 13].
According to the 2021 Practice Guideline of the American Association for the Study of Liver Diseases (AASLD), diagnostic laparocentesis should be performed in all patients with ascitic syndrome, even if they do not present obvious clinical signs of infection [14]. The diagnosis of spontaneous bacterial peritonitis in patients with liver cirrhosis and ascites is established based on both the general and bacteriological examination of the ascitic fluid [15].
The bacteriological examination of the ascitic fluid is performed before the initiation of empirical antibacterial therapy [15]. At the patient's bedside, at least 10 ml of ascitic fluid is collected and inoculated into a blood culture test tube, with evaluation done for both aerobic and anaerobic germs. At the same time, the patient's venous blood is collected for blood culture examination. The simultaneous collection of blood and ascitic fluid samples during the bacteriological examination increases the sensitivity of the method for detecting the microorganism involved in triggering SBP by 90% [16].
According to literature data, spontaneous bacterial peritonitis is typically a mono-bacterial infection, most frequently caused by gram-negative bacteria (in 60% of cases). The causative agent is usually specific to the intestinal flora, with Escherichia coli being the most common, followed by Klebsiella pneumoniae, Staphylococcus aureus, Enterococcus faecalis, and Enterococcus faecium [17]. However, there has been a recent increase in cases of SBP caused by gram-positive bacteria, including multidrug-resistant and quinolone-resistant strains. Gram-positive germs involved are Streptococcus spp., Enterococcus spp., and Staphylococcus spp. [18, 19].
Laparocentesis performed more than 48 hours after hospitalization, with the detection of SBP, is considered a nosocomial infection; multidrug-resistant bacteria are most frequently detected. Patients with decompensated liver cirrhosis, due to frequent hospitalizations and repeated exposure to invasive procedures and antibiotic administration, either for treatment or prophylaxis, often develop infections caused by multidrug-resistant bacteria, accounting for 35% of all infections [19].
With the initiation of antibacterial therapy and the prophylaxis of primary and recurrent SBP using norfloxacin, the detection of quinolone-resistant organisms in ascitic fluid has been increasingly reported, being attributed to nosocomial SBP [20].
Despite the use of high-sensitivity methods for detecting microorganisms in ascitic fluid, bacterial culture remains negative in up to 60% of cases of SBP confirmed by laparocentesis with neutrophils ≥250 cm3 [6].
According to the bacteriological result of the ascitic fluid and the presence of neutrophils we can differentiate:
spontaneous bacterial peritonitis (classic) - neutrophils in the ascitic fluid are ≥250/cm3, and the bacteriological examination is positive, which could be mono-bacterial or poly-bacterial.
neutrophilic spontaneous peritonitis, culture-negative – neutrophils in the ascitic fluid are ≥ 250/cm3, and the bacteriological examination is negative.
spontaneous bacterial peritonitis, or bacteriascites - neutrophils in the ascitic fluid are ≤ 250/mm3, and the bacterial culture of the ascitic fluid is positive [21].
In the group of patients investigated, the analysis of the protein level in the ascitic fluid showed that, in most cases, the protein level averaged 14 g/L. Runyon demonstrated that cirrhotic patients with protein levels in the ascitic fluid below 1 g/dL are 10 times more likely to develop SBP [22]. Thus, the antibacterial and opsonic activity of ascitic fluid is closely correlated with the protein concentration. Studies have confirmed that the ascetic fluid protein concentration is an essential predictor of the first episode of SBP [23, 24]. However, there were patients with a positive ascitic fluid culture confirming the diagnosis of SBP, but with high protein levels detected in the ascitic fluid, up to 47 g/L. These patients also presented with large fibrin deposits at laparoscopy.
Bacterial infection in patients with liver cirrhosis is the most frequent trigger of acute-on-chronic liver failure [25]. Acute-on-chronic liver failure in cirrhosis is a syndrome characterized by acute decompensation, organ failure, and high mortality [25, 26]. In the analyzed study group, mortality at 1 month was 8.5% (7 patients) due to progressive liver failure. Bacterial infections, especially spontaneous bacterial peritonitis, are a major problem and a significant prognostic factor in patients with acute-on-chronic liver failure [25, 26].
Conclusion
Spontaneous bacterial peritonitis has multiple variants and can be misdiagnosed due to the lack of specific signs and symptoms. In patients with ascites and cirrhosis, SBP may represent a source of latent abdominal sepsis. The laparoscopic approach enables both the diagnosis of latent spontaneous peritonitis and thorough peritoneal lavage, along with the installation of intra-abdominal drains and postoperative lavage of the abdominal cavity to improve peritoneal absorption function. Post-operative fractional lavage of the abdominal cavity facilitates better cleaning, enhancing peritoneal absorption. Thus, minimally invasive laparoscopic access constitutes a safe treatment option for patients with SBP and liver cirrhosis.
Competing interests
None declared.
Authors’ contributions
Study conception and design: GA, TZ. Data acquisition: GA, TZ, SP, OC, GL AA. Analysis and interpretation of data: GA, TZ. Drafting of the manuscript: TZ. Significant manuscript review with significant intellectual involvement: TZ, GA. Approval of the final version of the manuscript: GA, TZ, SP, OC, GL AA.
Acknowledgment
The research project was funded by the National Agency for Research and Development of the Republic of Moldova under the State Program 2020-2023 “Pathogenically proven new technologies in minimally invasive complex surgical laparo-endoscopic and endovascular treatment of patients with potentially lethal complications of cirrhosis”, No. 15.817.04.37A, Project Code - 20.80009.8007.30.
Patient consent
Obtained.
Ethics approval
The study was approved by the Research Ethics Committee of Nicolae Testemițanu State University of Medicine and Pharmacy - Minutes no. 15 from 28.02.2022.
Authors’ ORCID IDs
Gheorghe Anghelici – https://orcid.org/0009-0003-1063-2802
Tatiana Zugrav – https://orcid.org/0000-0002-5205-5471
Sergiu Pisarenco – https://orcid.org/0009-0003-6516-1630
Oleg Crudu – https://orcid.org/0009-0003-8616-7222
Gheorghe Lupu – https://orcid.org/0009-0004-0901-0341
Ana Apascaritei – https://orcid.org/0009-0007-3666-6794
References
Paraschiv A. Evoluția situației epidemiologice prin hepatite cronice, ciroze hepatice și cancer hepatic în Republica Moldova [Epidemiological evolution of chronic hepatitis, liver cirrhosis and liver cancer in the Republic of Moldova]. One Health Risck Manag (Chisinau). 2021;(2):83-92. doi: 10/38045/ohrm.2021.2.11. Romanian.
Li YT, Huang JR, Peng ML. Current status and prospects of spontaneous peritonitis in patients with cirrhosis. BioMed Res Int. 2020;1:1-12. doi: 10.1155/2020/3743962.
Fernandez J, Navasa M, Gomez J, et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfl oxacin prophylaxis. Hepatology. 2002;35(1):140-148.doi: 10.1053/jhep.2002.30082.
Fiore M, Maraolo AE, Gentile I, et al. Current concepts and future strategies in the antimicrobial therapy of emerging gram-positive spontaneous bacterial peritonitis. World J Hepatol. 2017;9(30):1166-1175. doi: 10.4254/wjh.v9.i30.1166.
European Association for the Study of the Liver; Paolo A, Mauro B, et al. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406-460. doi: 10.1016/j.jhep.2018.03.024.
Rimola A, García-Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, Inadomi JM. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32(1):142-53. doi: 10.1016/s0168-8278(00)80201-9.
Dumbrava VT, Țurcanu A, Lupașco I, Țurcan S, Hotineanu V, Cazacov V, Tofan-Scutaru L, Berliba E, Maximenco E; Ministry of Health of the Republic of Moldova. Ascita în ciroza hepatică la adult: Protocol clinic national [Ascites in liver cirrhosis in adults: National clinical protocol]. Chisinau: The Ministry; 2009. 44 p. Romanian.
Kim JJ, Tsukamoto MM, Mathur AK, Ghomri YM, Hou LA, Sheibani S, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. doi: 10.1038/ajg.2014.212.
Kasztelan-Szczerbinska B, Słomka M, Celinski K, Serwacki M, Szczerbinski M, Cichoz- Lach H. Prevalence of spontaneous bacterial peritonitis in asymptomatic inpatients with decompensated liver cirrhosis - a pilot study. Adv Med Sci. 2011;56(1):13-17. doi: 10.2478/v10039-011-0010-6.
Ruiz-Alcaraz AJ, Martinez-Banaclocha H, Marin-Sanchez P, Carmona-Martinez V, Iniesta-Albadalejo MA, Tristan-Manzano M, et al. Isolation of functional mature peritoneal macrophages from healthy humans. Immunol Cell Biol. 2020;98(2):114-126. doi: 10.1111/imcb.12305.
Wong CL, Holroyd-Leduc J, Thorpe KE, Straus SE. Does this patient have bacterial peritonitis or portal hypertension? How do I perform a paracentesis and analyze the results? JAMA. 2008;299(10):1166-1178.doi: 10.1001/jama.299.10.1166.
Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8(5):1151-1157.doi: 10.1002/hep.1840080532.
Such J, Francés R, Muñoz C, Zapater P, Casellas JA, et al. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology. 2002;36(1):135-141. doi: 10.1053/jhep.2002.33715.
Biggins SW, Angeli P, Garcia-Tsao G, Ginès P, Ling SC, Nadim MK, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the american association for the study of liver diseases. Hepatology. 2021;74(2):1014-1048. doi: 10.1002/hep.31884.
Aithal GP, Palaniyappan N, China L, Härmälä S, Macken L, Ryan JM, et al. Guidelines on the management of ascites in cirrhosis. Gut. 2021;70(1):9-29. doi: 10.1136/gutjnl-2020-321790.
Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95(5):1351-1355. doi: 10.1016/0016-5085(88)90372-1.
Piano S, Singh V, Caraceni P, Maiwall R, Alessandria C, Fernandez J, et al. Epidemiology and effects of bacterial infections in patients with cirrhosis worldwide. Gastroenterology. 2019;156(5):1368-1380.e10. doi: 10.1053/j.gastro.2018.12.005.
Dunn DL, Barke RA, Knight NB, Humphrey EW, Simmons RL. Role of resident macrophages, peripheral neutrophils, and translymphatic absorption in bacterial clearance from the peritoneal cavity. Infect Immun. 1985;49(2):257-264. doi: 10.1128/iai.49.2.257-264.1985.
Tsiaoussis GI, Assimakopoulos SF, Tsamandas AC, Triantos CK, Thomopoulos KC. Intestinal barrier dysfunction in cirrhosis: Current concepts in pathophysiology and clinical implications. World J Hepatol. 2015;7(17):2058-2068. doi: 10.4254/wjh.v7.i17.2058.
Piano S, Fasolato S, Salinas F, Romano A, Tonon M, Morando F, et al. The empirical antibiotic treatment of nosocomial spontaneous bacterial peritonitis: Results of a randomized, controlled clinical trial. Hepatology. 2016;63(4):1299-1309. doi: 10.1002/hep.27941.
Huang CH, Lee CH, Chang C. Spontaneous bacterial peritonitis in decompensated liver cirrhosis: a literature review. Livers. 2022;(2):214-232. https://doi.org/10.3390/livers2030018.
Runyon BA. Low-protein-concentration ascitic fluid is predisposed to spontaneous bacterial peritonitis. Gastroenterology. 1986;91(6):1343-1346. doi: 10.1016/0016-5085(86)90185-x.
Fernández J, Acevedo J, Castro M, Garcia O, de Lope CR, Roca D, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology 2012;55(5):1551-1561. doi: 10.1002/hep.25532.
Parsi MA, Saadeh SN, Zein NN, Davis GL, Lopez R, Boone J, et al. Ascitic fluid lactoferrin for diagnosis of spontaneous bacterial peritonitis. Gastroenterology. 2008;135(3):803-7.doi: 10.1053/j.gastro.2008.05.045.
Fernández J, Acevedo J, Wiest R, et al.; European Foundation for the Study of Chronic Liver Failure. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. 2018;67(10):1870-1880. doi: 10.1136/gutjnl-2017-314240.
Garcia-Tsao G. Ascites. In: Dooley JS, Lok ASF, Burroughs AK, Heathcote EJ, editors. Sherlock’s diseases of the liver and biliary system. 12th ed. Cham: Wiley-Blackwell; 2011. p. 210-133.