Introduction
Epilepsy affects approximately 50 million people worldwide. One-third of them show drug resistance to antiepileptic drugs [1-3]. Specific biomarkers essential to determine drug resistance are not currently established. The biomarkers are defined as „cellular, biochemical or molecular changes, which can be measured in biological environments, such as human tissues, cells or fluids” [4, 5]. More recently, a working group of the National Institutes of Health (NIH) extended this definition to „a characteristic that can be objectively measured and that is an indicator of normal and pathological biological processes or pharmacological response to applied therapeutic intervention” [6]. In the case of epilepsy, the spectrum of biomarkers ranges from neuroimaging and electrophysiological markers to molecular and cellular markers, determined in peripheral fluids and tissues.
This article is a review article on soluble biomarkers, determined in blood and cerebrospinal fluid (CSF). They may have multiple potential uses such as the ability to predict the development of epilepsy following a brain injury and/or after a first seizure, the progression of the disease and its severity, and the possibility of developing drug resistance to antiepileptic drugs [4]. Thus, bibliographic sources were analyzed in which data related to the importance of biomarkers in epilepsy were exposed.
It is known that the biomarkers of epileptogenesis are expensive and difficult to research. Even after severe brain conditions, in the presence of a potential epileptogenic risk, such as severe head trauma, the proportion of people who develop epilepsy is not high. Moreover, this process can take decades. An ideal situation would be to identify the biomarkers specific to the entire epileptogenic process, in close proximity to neuronal injury, with the possibility of predicting the risk of developing seizures, epilepsy, and resistance to treatment [7, 8].
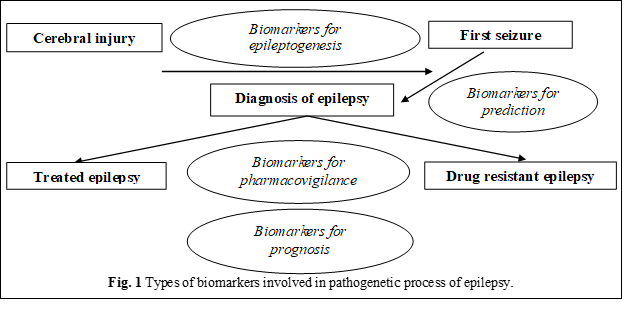
Molecular and cellular biomarkers should ideally be present in an accessible compartment such as blood, tissues, CSF, sputum, or urine (Figure 1) [9].
Material and methods
Publications on diagnostic biomarkers of epilepsy were reviewed. References were identified by a search in the PubMed, MEDLINE and Scopus databases for articles published until June 2022, with various combinations of the terms – „epilepsy”, „seizures”, „epileptogenesis”, „biomarkers”, „neuroimaging”, „inflammation”, „status epilepticus”, „prognosis”. A qualitative and analytical study was performed focused on primary studies published between 2020-2022 and dedicated to the identification of biomarkers for diagnosis, disease progression, and complications of epilepsy. The articles contain a detailed analysis and a synthesis of recommendations regarding the targeted selection of biomarkers in the diagnosis of epilepsy. Articles were selected taking into consideration the title and their abstracts. More than 85 sources were identified and 33 were selected for analysis. 12 articles, 5 clinical trials, 2 meta-analyses, 7 reviews, and 7 systematic reviews were identified.
Results and discussion
Inflammation
Recent experimental studies have revealed that inflammation can precipitate seizures and support convulsive activity [10-12]. In addition, it can influence epileptic discharge by changes in glutamate and ions homeostasis involved in the epileptogenic process. Consequently, biological markers of inflammation represent a pathway for identifying patients in whom inflammation plays a key role in epileptogenesis and/or maintaining neuronal excitability [10, 11]. Furthermore, immunomodulatory drugs, including steroids and intravenous immunoglobulins, have been shown to have successful therapeutic effects in some children with epileptic encephalopathies, which are often resistant to conventional antiepileptic drugs (AED).
Inflammation of the tissues can be involved not only in the generation of seizures but also in the development of the drug resistance phenotype. Surprisingly, even children with focal seizures, seizures that are not traditionally considered to be inflammatory in nature, have a positive response to steroids. Targeting the inflammatory process may present a novel therapeutic strategy in the treatment of epilepsy and circulating biomarkers capable of demonstrating the response to treatment have an increased value [12, 13].
In several studies, individuals with drug-resistant focal epilepsy have been shown to exhibit an imbalance in the ratio of interleukin-1 / interleukin-1 receptor antagonists (IL-1/IL-1RA). IL-1 is recognized as a mediator of brain inflammation. In rodents, pharmacological blocking of IL-1 biosynthesis significantly reduces the risk of developing seizures. This “pro-inflammatory cytokine profile” in peripheral blood, consisting of elevated IL-6 levels with a low IL-1/IL-1RA ratio, may be found among patients in whom persistent and unsolved inflammation leads to the development of neuromodulation associated with changes in neuronal excitability. Likewise, a higher concentration of IL1-Elm in serum and CSF was associated with an increased risk of developing epilepsy after brain damage [14].
The high-mobility group box-1 (HMGB1) is one of the best-known mediators of neuroinflammation evoked by epileptogenic lesions, a fact proven in clinical models of epilepsy [15]. It is actively released by immune cells during injury-induced infection or aseptic inflammation. Cellular death leads to the passive release of HMGB1. Experimental models of epilepsy suggest that the acetylated disulphide form of HMGB1 is responsible for the occurrence of inflammation in epilepsy. A pilot study in patients with resistant focal epilepsy, suggests that HMGB1 isoforms can be considered candidate biomarkers in epilepsy stratification [16]. However, HMGB1 is not only specific for epilepsy and in fact, it is a sensitive and specific biomarker for several conditions, including autoimmune pathologies and malignancies.
Pharmacological inhibition of HMGB1 has been successful in numerous experimental disease models [17]. Interventions used included direct inhibition using polyclonal and monoclonal antibodies, competitive HMGB1 a-Box inhibitors, HMGB1 sequestration, and degradation methods. Since HMGB1 has no cerebral specificity, it is unclear whether peripheral or central nervous system (CNS) changes are responsible for seizures, or the therapeutic effects described above.
Blood-brain barrier
Dysfunction of the blood-brain barrier following prolonged seizures in animal models has been recognized since the 1950s. Vasogenic edema, caused by neurovascular changes, was first described by Klatzo and colleagues. In some cases, dysfunction of the blood-brain barrier can cause seizures, while dysfunction created artificially by other means leads to delayed epileptogenesis [18, 19]. Impaired blood-brain barrier function due to a hypertensive crisis in eclampsia and hypertensive encephalopathy may involve changes in serum magnesium levels. Injury to the blood-brain barrier in experimental studies caused the delayed occurrence of seizures [20, 21]. Impaired blood-brain barrier function is commonly found in various neurological conditions, such as encephalitis, meningitis, stroke, Alzheimer's disease, and other CNS disorders. There is no doubt that cerebrovascular dysfunction conditions or sustains seizures. The role of cerebrovascular impairment in various CNS disorders, including epilepsy, has been clinically accepted in the past, but only recently, it has been tested as an important mechanism underlying epileptogenesis. In the case of human epilepsy, the results of the studies are suggestive for a loss of selective permeability of the blood-brain barrier in the focal regions from which the convulsions originate [22, 23].
In addition, data from several studies support the idea that the blood-brain barrier in patients with epilepsy exhibits a variety of molecular changes that are in one way or another involved in epileptic disease [18-20]. Given the importance of the blood-brain barrier for convulsive disorders and epilepsy, it is not surprising that biomarkers in this pathophysiological field are highly examined, but at the same time, they require further research. In general, there are three approaches to determine the integrity of the blood-brain barrier in epilepsy. They do not differ from aspects previously used to measure cerebrovascular integrity in other neurological diseases [7]. Historically, the ratio of serum albumin to CSF albumin was the first used approach. It is known that the vascular barrier protects the brain from harmful substances in the blood while providing the necessary nutrients to the brain for proper functioning and strict regulation of the traffic of cells and molecules from the blood to the brain. The vascular barrier also separates impermeable macromolecules (>~500 Da) in the blood and brain. Thus, when the blood-brain barrier is intact, the albumin is 10 times more concentrated in the blood and will have a constant ratio to the concentration in the CSF [20]. A similar principle, but applied in another way, would be the nuclear magnetic resonance imaging (MRI) with the application of contrast agents. In this case, the „ratio” of the cerebral parenchyma to the blood is measured topographically, and the distribution of the substance injected into the blood is visualized in the brain. The absence of extravasation indicates an intact blood-brain barrier. Thus, the brain produces specific proteins that are isolated in the CNS in conditions of the intact blood-brain barrier. In there is a lesion of the blood-brain barrier, proteins, which are normally present in high concentrations in the CNS, are free to diffuse into the blood according to their concentration gradient. An ideal peripheral biomarker of clinical significance, should be: (1) a protein (or molecule) present in small or undetectable concentrations in the serum of healthy subjects; (2) present in the brain and CSF, being in a higher concentration in the cerebral parenchyma than in plasma; (3) available for extravasation in case of damage to the blood-brain barrier; and, (4) further released by brain cells in response to brain damage (for example, during reactive gliosis). Several proteins, including S100 calcium-binding protein B (S100B), neuronal-specific enolase (NSE), and glial fibrillary protein (GFAP) have been evaluated for their functions, and the S100B protein meets all the above-mentioned criteria. The imaging techniques available for clinical research do not have the high resolution required to „visualize” this structure, although the contrast agents that have been used to measure the integrity of the blood-brain barrier. Functional assessment of the status of the blood-brain barrier by calculating the albumin coefficient (QA) in serum and CSF and MRI with contrast is widely accepted as the gold standard for determining the integrity of the blood-brain barrier. Recent work has shown that the serum level of the S100B protein correlates with QA, thus allowing the measurement of the CSF protein indirectly and without performing a lumbar puncture [21, 22].
It is relevant that serum S100B protein is the most studied marker, which may be involved in the mechanisms of delayed epileptogenesis after traumatic brain injury. Brain injury, in general, is associated with a rapid loss of integrity of the blood-brain barrier followed by the development of brain disease. Thus, the protein S100B emerged as a peripheral biomarker indicative of the permeability of the blood-brain barrier. The increase in serum levels of S100B reflects the presence of a damaged barrier and can predict long-term consequences after a brain pathology. Intracranial events are associated with an increased risk of seizures; thus, it is possible that the S100B protein will also prove to have a utility in detecting people at increased risk for seizures. Two independent meta-analyses for S100B in traumatic brain injury concluded that normal S100B levels accurately predict the results of a normal neuroimaging exam and that S100B sampling within 3 hours of injury should be considered when there is no focal neurological deficit or significant extracerebral injury [24, 25]. Therefore, there is an opportunity to conduct a test with a high predictive level, so that many unnecessary scans can be avoided, a test with a predictive value to diagnose complications after brain trauma.
CCL-2 is a pro-inflammatory chemokine produced by hyperactive neurons and microglia, it is involved in immune cell migration, chemoattraction of monocytic cells, disruption of blood-brain barrier and neuroinflammation. Its production is stimulated by IL-1β and tumor necrosis factor α (TNF-α) in the microglia, vascular endothelial cells, pericytes and neurons. The activation of CCL2 initiates the adhesion of monocytes to the inflamed endothelium, resulting in infiltration into the brain parenchyma [26, 27].
Aquaporin 4 (AQP4) is a membrane protein, serving as a water channel in conformity to the osmotic gradient; it is expressed by glial cells in the brain and the spinal cord. These channels are localized in the astrocytic plasma membrane that abut on brain micro vessels or on the pial covering of the brain surface. Both lowered expression and incorrect localization of AQP4 on end-foot membranes can lead to an alteration in the astrocytic function and homeostasis. The astrocytic AQP4 channels mediate water clearance in vasogenic edema with exacerbation of intracellular edema and so lead to cellular edema in the CNS. It was found that abnormalities in astrocytic ion channel expression, localization, and function could cause alteration of ion homeostasis and neurotransmitters, deposition of characteristic proteins, oxidative stress, and neuroinflammation. The overexpression and altered localization of AQP4 channels in astrocytes has been suggested as a reason for blood–brain barrier (BBB) dysfunction, manifested by the reduction in the flow of brain interstitial fluid mediated by AQP4 and due to a failure of clearance accumulation of pathologic markers such as amyloid-β (Aβ), and mutant α-synuclein (α-SYN) which trigger the production of reactive oxygen species (ROS) and inflammatory factors and contributes to the pathological progression of neurodegenerative diseases [28, 29].
Scientific evidence maintains the hypothesis that brain tissue inflammation is a central component for the development of seizures. Toll-like receptors (TLR) 4 is a trans-membrane protein that is highly expressed in the neurons, astrocytes, and microglia under pathological situations, such as cell injury and epileptic seizure [30, 31]. A study suggests a significant association between homozygous CC and heterozygous CT genotypes of TLR4 and epilepsy. It identifies these genotypes as a risk factor for epilepsy [32]. Some studies in rat models suggest that TLR4 signaling takes part in the drug-resistant epilepsy pathway. Inhibition of TLR4 downregulates IL-1β, TNF-α and NF-κB at the cellular level. The same study reveals that inflammatory pathways in drug-resistant epilepsy are activated. Such inflammatory factors as TLR4, IL-1β, TNF-α, NF-κB signaling factors and P-glycoprotein seem to be involved in the genesis of a drug-resistant epilepsy network. TLR4 was proposed to be an upstream regulator, that activates the downstream NF-κB, regulates inflammatory factors IL-1β, TNF-α, and other cytokines [33].
Conclusions
Epilepsy is represented by a research area of specific biomarkers, which are of great clinical importance, being necessary for forecasting the disease, the risk of developing neurological sequelae, and refractory to antiepileptic remedies. Thus, their identification could have a significant impact on the clinical course of the disease. It should be recognized that many of the biomarkers discussed in this review are also involved in other pathologies, including non-neurological diseases. Prospective research will be needed to identify individual or groups of specific biomarkers that would differentiate epilepsies from other diseases. New therapeutic strategies should involve integrating clinical information, including electroencephalography and neuroimaging exams, with new molecular and cellular biomarkers.
Identifying biomarkers of aberrant inflammation could stratify patients to determine who contributes to maintaining epileptic status. Focusing on inflammation, which plays a critical role in epilepsy may encourage the development of various strategies that halt the progression of the drug-resistant phenotype.
Markers of the integrity of the blood-brain barrier are useful tools for determining sequels in a variety of neurological diseases or acute events (strokes, trauma). Currently, the role of these markers in the prognosis and diagnosis of convulsive disorders is being studied, although, from, another point of view, these markers have already demonstrated that the blood-brain barrier is damaged now of convulsive seizures and that the disruption of the integrity of this barrier is epileptogenic. Moreover, when the blood-brain barrier is damaged, allowing albumin to enter the interstitial space, can affect the effectiveness of some drugs and, therefore, markers of barrier integrity can be useful in therapeutic decision-making.
Better tools to predict the onset of epilepsy could lead to the development of new therapeutic strategies to prevent the development of epilepsy, potentially in the form of immunomodulatory intervention. In addition, early prediction of drug resistance would mean that patients could be evaluated for epilepsy surgery at an early stage, thus avoiding the administration of more antiepileptic drugs that are associated with side effects, are inevitably doomed to fail.
Competing interests
None declared.
Authors’ contribution
CC, IC, AC, LF, OC – the conception and the design of the study, the acquisition of data, the drafting of the manuscript; SH, SG – the critical revising of the manuscript for important intellectual content and the approval of the version of the manuscript to be published. All the authors – the analysis and the interpretation of data.
Funding
This study was supported by the Nicolae Testemitanu State University of Medicine and Pharmacy, and the research project within the state program „Integrating the mechanisms of epileptogenesis to create the network of multimodal diagnosis and treatment of epilepsy” – 20.80009.8007.40, offered by the National Agency for Research and Development of the Government of the Republic of Moldova.
Authors’ ORCID IDs
Cornelia Călcîi - https://orcid.org/0000-0002-2608-2417
Ludmila Feghiu - https://orcid.org/0000-0002-1425-1885
Svetlana Hadjiu - https://orcid.org/0000-0001-5281-3626
Stanislav Groppa - https://orcid.org/0000-0002-2120-2408
References
Berg A.T., Berkovic S.F., Brodie M.J. et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia, 2010; 51:676-685.
Engel J. Jr. International League Against Epilepsy. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia, 2001; 42:796-803.
PitkanenA. Drug-mediated neuroprotection and antiepileptogenesis: animal data. Neurology, 2002; 59: S27-S33.
Marchi N., Cavaglia M., Fazio V. et al. Peripheral markers of blood brain barrier damage. Clin. Chim. Acta, 2004; 342:1-12.
Mao L.Y., Ding J., Peng W.F. et al. Interictal interleukin-17A levels are elevated and correlate with seizure severity of epilepsy patients. Epilepsia, 2013; 54: e142-e145.
Maroso M., Balosso S., Ravizza T. et al. Toll-like receptor 4 and high mobility group box-1are involved in ictogenesis and can be targeted to reduce seizures. Nat.Med, 2010; 16:413-419.
Masel B.E., DeWitt D.S. Traumatic brain injury: a disease process, not an event. J. Neurotrauma, 2010; 27:1529-1540.
Oby E., Janigro D. The blood-brain barrier and epilepsy. Epilepsia, 2006; 47:1761-1774.
Lauren E. Walker, Damir Janigro, Uwe Heinemann, Raili Riikonen, Christophe Bernard, and Manisha Patel. Molecular and cellular biomarkers for epilepsy. Epilepsia,2016; 57(9):1354–1362.
Andersson U., Tracey K.J. HMGB1 is a therapeutic target for sterile inflammation and infection. Ann. Rev. Immunol, 2011; 29:139-162.
Diamond M.L., Ritter A.C., Failla M.D. et al. IL-1beta associations with posttraumatic epilepsy development: a genetics and biomarker cohort study. Epilepsia, 2014; 55:1109-1119.
de Vries H.E., Kooij G., Frenkel D. et al. Inflammatory events at blood-brain barrier in neuroinflammatory and neurodegenerative disorders: implications for clinical disease. Epilepsia, 2012; 53 (Suppl.6):45-52.
Lehtimaki K.A., LiimatainenS., PeltolaJ. et al. The serum level of interleukin-6 in patients with intellectual disability and refractory epilepsy. Epilepsy Res., 2011; 95:184-187.
Maroso M., Balosso S., Ravizza T. et al. Interleukin-1beta biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics, 2011; 8: 304-315.
Balosso S., Liu J., Bianchi M.E. et al. Disulphide-containing high mobility group box-1 promotes N-Methyl-d-aspartate receptor function and excitotoxicity by activating toll-like receptor 4-dependent signaling in hippocampal neurons. Antioxid. Redox Signal, 2014; 21(12):1726-40.
Musumeci D., Roviello G.N., Montesarchio D. An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies. Pharmacol.Ther. 2014; 141: 347-357.
Pilzweger C., Holdenrieder S. Circulating HMGB1and RAGE as clinical biomarkers in malignant and autoimmune diseases. Diagnostics (Basel), 2015; 5:219-253.
Blyth B.J., Farhavar A., Gee C. et al. Validation of serum markers for blood-brain barrier disruption in traumatic brain injury. J. Neurotrauma, 2009; 26:1497-1507.
Gorter J.A., van Vliet E.A., Aronica E. Status epilepticus, blood-brain barrier disruption, inflammation, and epileptogenesis. Epilepsy Behav. 2015; 49:13-16.
Hoffmann A., Bredno J., Wendland M.F. et al. Validation of in vivo magnetic resonance imaging blood-brain barrier permeability measurements by comparison with gold standard histology. Stroke, 2011; 42:2054-2060.
JanigroD. Are you in or out? Leukocyte, ion, and neurotransmitter permeability across the epileptic blood-brain barrier. Epilepsia, 2012; 53 (Suppl.1):26-34.
Marchi N., Granata T., Ghosh C. et al. Blood-brain barrier dysfunction and epilepsy: pathophysiologic role and therapeutic approaches. Epilepsia, 2012; 53:1877-1886.
Marchi N., Angelov L., Masaryk T. et al. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia,2007; 48:732-742.
Heidari K., Vafaee A., Rastekenari A.M. et al. S100B protein as a screening tool for computed tomography findings after mild traumatic brain injury. Systematic review and meta-analysis. Brain Inj. 2015; 11:1-12.
KapuralM., Krizanac-BengezL., BarnettG. et al. SerumS-100 beta possible marker of blood-brain barrier disruption. Brain Res. 2002, 940:102-104.
Bozzi Y., Caleo M. Epilepsy, Seizures, and Inflammation: Role of the C-C Motif Ligand 2 Chemokine. DNA Cell. Biol. 2016; 35:257-260.
Tian D.S., Peng J., Murugan M. et al. Chemokine CCL2-CCR2 Signalling Induces Neuronal Cell Death via STAT3 Activation and IL-1β Production after Status Epilepticus. J. Neurosci., 2017; 37, 7878-7892.
Glober N.K., Sprague S., Ahmad S. et al. Acetazolamide treatment prevents redistribution of astrocyte Aquaporin 4 after murine traumatic brain injury. Neurosci. J., 2019:2831501.
Harrison I.F., Ismail O., Machhada A. et al. Impaired lymphatic function and clearance of tau in an Alzheimer’s disease model. Brain, 2020; 143, 2576-2593.
FrigerioF. et al. Neuroinflammation alters integrative properties of rat hippocampal pyramidal cellsMol. Neurobiol. 2018; 49:13-16.
LiaoE. et al. Electric stimulation of ear reduces the effect of toll-like receptor 4 signaling pathway on kainic acid-induced epileptic seizures in rats. Biomed. Res. Int, (2018).
Abdelsalam M., AbdElmagid D., Magdy H. et al. The association between toll-like receptor (TLR4) genotyping and the risk of epilepsy in children. Egypt J. Med. Hum. Genet. 2020; 21, 6136.
XinghuaTanga., Xiaoxia Chena. The TLR4 mediated inflammatory signal pathway might be involved in drug resistance in drug-resistant epileptic rats. Journal of Neuroimmunology. 2022; Vol. 365, 577802.