Introduction
Regardless of the type of surgery, the post-operative period is a critical one, during which many complications can occur, such as acute post-operative pain, respiratory and/or hemodynamic instability, nausea and post-operative vomiting. Postoperative urinary retention (POUR) is one of the complications that can develop in the first 24 hours after surgery and often remains unrecognized and underestimated. Essentially, POUR is the inability to urinate in the presence of a full bladder in the early postoperative period. The prevalence of the complication varies widely, from 5 to 70% which indicates on the one hand a lack of consensus on clear criteria for POUR, and on the other hand confirming the multitude of risk factors related to this complication [1, 2]. Over the years, several risk factors associated with POUR have been studied and reported: unmodifiable (male [2-4], female [4-7], age [1, 2, 4, 8-10], comorbidities (diabetes mellitus (DM) [1, 2, 4], pre-existing neurological disorders [2, 4, 10]) and modifiable, related to the type of surgery (anorectal, colorectal, urogenital surgery [1, 2, 4, 6, 8]) and duration of surgical treatment (consisting of surgery under the protection of a certain type of anesthesia) [2-4, 10], intraoperative fluid volume [1, 2, 4, 6, 10], medication used (opioids [4, 8, 10], anticholinergics and antipsychotics [10]) and high American Society of Anesthesiologists score (ASA) [9], among others .
If left undiagnosed and untreated in a timely manner, POUR can lead to increased morbidity due to urinary tract infections, detrusor muscle dysfunction, arrhythmias and delirium, with prolonged hospital stay [2]. These complications associated with POUR have increased attention on early diagnosis of the phenomenon with the development of prevention strategies. Recently, preventive ultrasound diagnosis of POUR has gained significant importance [11].
The perioperative period may affect micturition with precipitation of POUR. As a spinal reflex controlled by the brainstem, the urination process is a complex one, consisting of 2 phases - the storage phase (mediated by sympathetic innervation) and the emptying phase (provided by parasympathetic fibers). The bladder itself is a container with a flexible muscular wall, which can hold an increasing volume of urine without large variations in pressure until a certain threshold is reached. On average, normal bladder capacity is 400-600 ml [10]. The first impulse to urinate occurs when the bladder volume is about 150 ml, and the sensation of fullness occurs at 300 ml volume, which, once exceeded, transmits the information through the pelvic splanchnic system, e activating emptying process by parasympathetic fibers [4]. For bladder emptying to occur, inhibition of the motor cortex must be absent and the contraction of the detrusor muscle. Motor cortex disinhibition is triggered by pudendal afferents as soon as urine enters the posterior urethra. As a result, relaxation of the pelvic floor muscle, descent of the levator ani muscle and voiding of urine occurs [1, 4, 10].
Surgical and intra-anesthetic stressor factors, acute postoperative pain, medications used, and patient comorbidities can complexly interfere with the physiological pathways of micturition, resulting in POUR [3, 5, 8, 10]. For instance, opioids may alleviate the sensation of bladder fullness through parasympathetic inhibition and increased sphincter tone due to increased sympathetic activity [4, 5, 10]. Beyond smooth muscle relaxation with reduced bladder contractility, multimodal general anesthesia also predisposes to POUR by causing autonomic bladder tone dysregulation [3-5]. Neuraxial techniques interfere with both the afferent and efferent pathways of micturition, with the prevalence of POUR being directly proportional to the duration of action of the local anesthetic molecule introduced intrathecally [2-5, 10]. Neuraxial opioids are associated with an increased prevalence of POUR compared with their intravenous administration [4].
The study aimed to estimate the prevalence of POUR in a medical institution with a surgical profile from Moldova and to identify a series of risk factors.
Material and methods
Study design and parameters
The prospective, observational, monocentric, cohort study, designed to elucidate the prevalence and characteristics of POUR in a medical institution in Moldova, was conducted from June 1, 2022, to December 31, 2022, at Valeriu Ghereg Anesthesiology and Resuscitation Department No.1, and the Institute of Emergency Medicine. All included patients signed informed consent to participate in the study.
Participants
A number of surgical patients were evaluated weekly according to eligibility criteria. The inclusion criteria were age > 18 years, signed informed consent; undergoing general, neuroaxial, locoregional or combined type of anesthesia; surgery lasting > 30 minutes, ASA score I - III. Patients were excluded if they expressed their wish to leave the study, positive history of renal failure, benign prostatic hypertrophy with obstruction, required bladder catheterization from the beginning of the surgery or lasting surgery > 3 hours or were scheduled for ambulatory surgery.
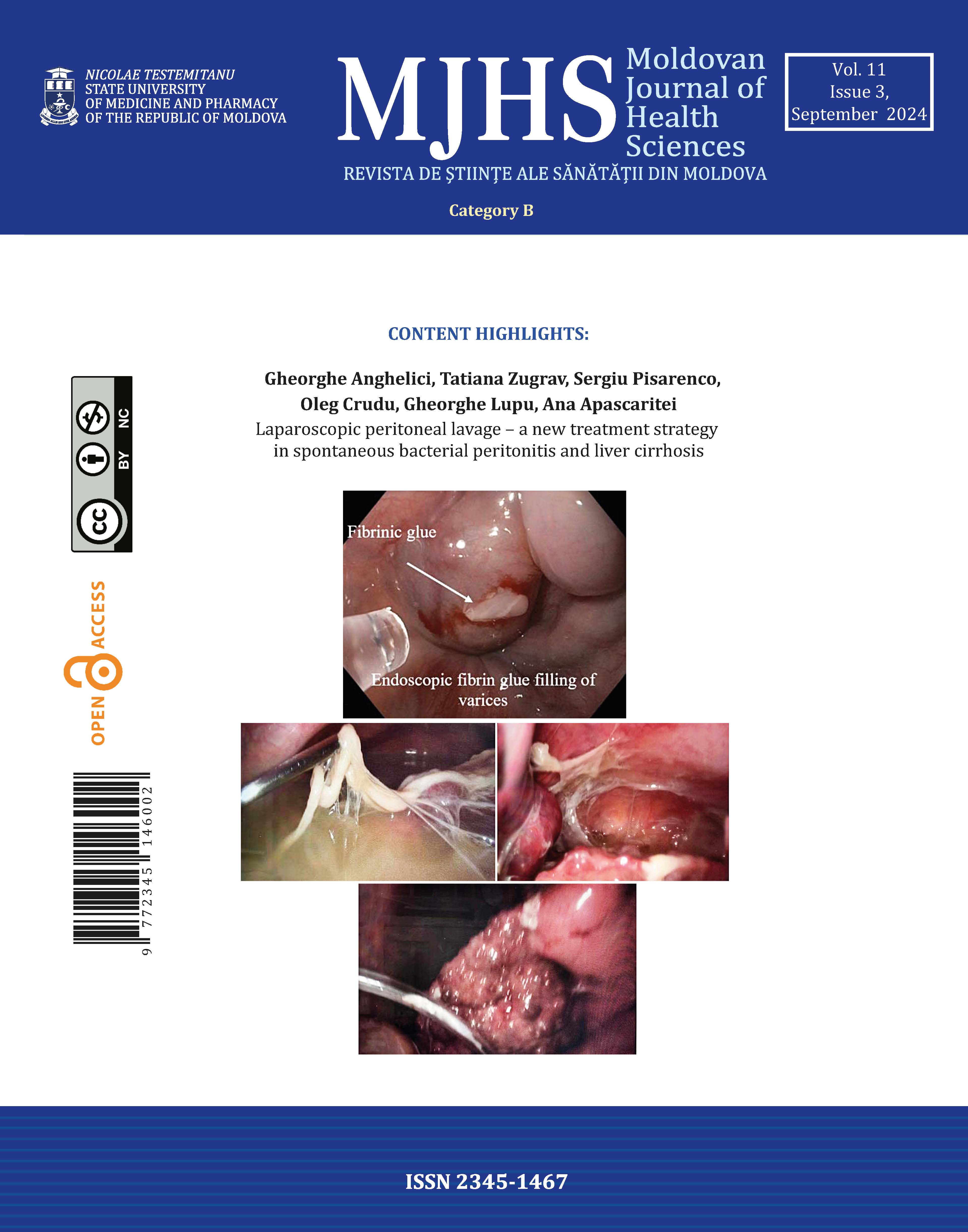
The Consort flow chart with the pattern of refusals, enrolment, and follow-up in the first 24 hours postoperatively is shown in Figure 1.
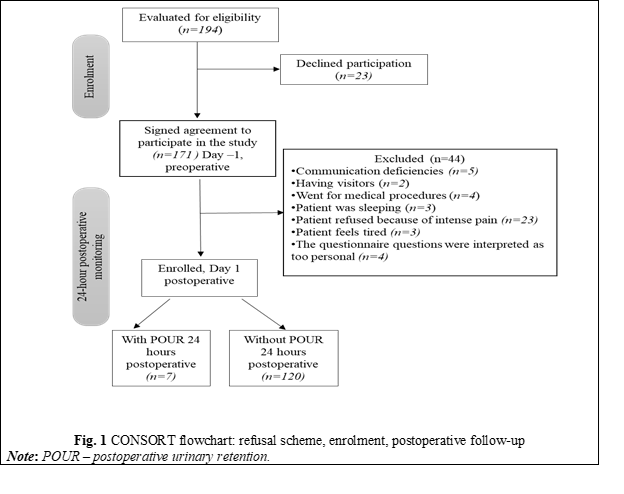
After obtaining informed consent to participate, the study questionnaire, developed based on the literature review, was completed with the patient's personal and clinical data regarding risk factors for POUR. The questionnaire included 3 compartments: general patient data (comorbidities, chronic medication, surgical profile), data related to peri-anesthesic management and a stipulation of study’s inclusion and exclusion criteria. The time points of recording the parameters are represented in the study diagram (Figure 2).
Recorded parameters and statistical analysis
The following general parameters were recorded: age, sex, height, body mass, type of surgery, duration of anesthesia, duration of surgery; and parameters tested as risk factors: preoperative, intraoperative and postoperative factors, directly related to patient or the medical act itself and the medication administered - their nominal detail are presented in the results. The values of the parameters were numerated via de-identification in an Excel table and then imported into the statistical analysis software Social Science Statistics (https://www.socscistatistics.com). Data are presented as absolute and relative values or mean and standard deviation. The Fisher exact test, the hi-squared independence test (X2) including Yates correction, odds ratio (OR) were calculated, and a p˂0.05 was considered statistically significant.
All respondents agreed to participate in the research and signed the informed consent for participation in the study, as well as the informed consents for hospitalization, surgery and anesthesia. The present study observes individuals and measures variables of interest but does not attempt to influence the treatment, lacking randomization of exposure. Also, the observational data are reported in grouped format (counts and percentages), ensuring that participants cannot be identified from the results. The nature of the data reported in the manuscript is not of a sensitive nature, and the study is generally considered a low-risk project.
Results
The general characterization of the 127 patients enrolled in the study is presented in Table 1. The mean age of the patients was 47.0±19 years (ranging from 18 to 83 years). The mean age of patients who did not develop POUR versus those who developed POUR was 45.8±18.6 and 60.0±10.9 (p = 0.951) years, respectively. The surgical population studied was homogeneous in terms of anthropometric criteria and heterogeneous in terms of gender distribution, with a predominance of males.
Table 1. General characterization of patients enrolled in the study. | ||||
Parameter | Enrolled (n = 127) | Non-POUR (n = 120) | POUR* (n = 7) | No difference |
Age, years | 47.0±19 (18 – 83) | 45.8±18.6 (18 – 83) | 60.0±10,9 (42 – 70) |
|
18 – 24 | 19 (15.0 %) | 19(15.8 %) | 0 (0 %) | |
25 – 44 | 41 (32.3 %) | 40 (33.4 %) | 1 (14.3%) | |
45 – 64 | 35 (27.5 %) | 33 (27.5 %) | 2 (28.6%) | |
>65 | 32 (25.2 %) | 28 (23.3 %) | 4 (57.1 %) | |
Men/ Women | 48/ (37.8 %), 1.6/1 | 76 (63.3 %)/ 44 (36.7 %), 1.7/1 | 3 (42.9 %)/ 4 (57.1%), 0.75/1 | X2 = 1.046 p* = 0.306 |
Body mass, kg | 77.9 (50 – 108) | 77.91 (50 – 108) | 76.8 (60 – 101) | t = 0.176 p* = 0.860 |
BMI<25 | 47 (37 %) | 45 (37.5 %) | 2 (28.6 %) | X2 = 0.226 p* = 0.942 |
BMI>25 | 80 (63 %) | 75 (62.5 %) | 5 (71.4 %) | |
Height, cm | 173.8 (157 – 192.0) | 174.0 (157 – 192) | 170.3 (160 – 187) | t = 0.331 p* = 0.740 |
Note: data are presented as absolute and relative values, mean and standard deviation, POUR - acute postoperative urinary retention (POUR* - where POUR was considered the inability to urinate in the presence of bladder globus accompanied by specific clinical signs with the need for bladder catheterization [1]); BMI – body mass index, *p < 0.05 statistically significant, t – T-student test. | ||||
The surgical procedures included: abdominal surgery - appendectomy (34/127 [26.8%]), cholecystectomy (6/127 [4.7%]), inguinal/ventral hernioplasty (8/127 [6.3%]), laparotomies for acute abdomen (15/127 [15.8%])); pelvic surgery (3/127 [2.35%]); traumatology surgery - upper/ lower limb (25/127 [40.9%], of which 11 cases of septic trauma); thoracotomies (3,127 [2.35%]; neurosurgery (3/127 [2.35%]); oromaxillofacial surgery (3/127 [2.35%]). The average duration of anesthesia was 122.1±31.4 minutes and the average duration of surgery was 111.3±30.3 minutes. The group comprised 3 types of anesthesia: general anesthesia 64% (81/127), spinal anesthesia 26% (33/127), peripheral nerve block 10% (13/127).
In our study, we identified a POUR prevalence of 5.5%. Patients with ASA IV-V anesthetic risk, who have severe preoperative comorbidities, were not included in the present study. As a result, this limitation will not allow us to generalize the obtained POUR prevalence. In addition, the exclusion of patients with ASA IV-V anesthetic risk also eliminates the risk of overestimating POUR prevalence.
The literature reports a wide range of POUR prevalence, from 5% to 70% [2]. The large discrepancy between the reported prevalence may indicate the complexity and multitude of risk factors contributing to the development of POUR on the one hand, and on the other hand, the heterogeneity of criteria used to diagnose POUR. Thus, in the present study, POUR was defined as the postoperative clinical situation characterized by inability to urinate requiring bladder catheterization. However, Cataldo P. [12] and Paulsen E. [13] establish the diagnosis of POUR even in the absence of micturition for ≥ 8 hours postoperatively. In addition to patients who required the insertion of Foley catheter, we recorded 3 patients who reported their first voiding at ≥ 8 hours after surgery (8, 13 and 9.5 hours), which would contribute to an increased prevalence of POUR in the present study from 5.5% (7/127) to 7.9% (10/127). Dobbs S. defines POUR when patient is unable to urinate for ≥12 hours postoperatively [14]. Applying this criterion in our study, POUR would have a prevalence of 6.3% (8/127) (Table 2).
Table 2. Prevalence of postoperative urinary retention depending on the definition criteria. | ||
Postoperative urinary retention definition criteria | International studies | Moldova (present study) |
Bladder catheterization [1] | 5 -70% | 5,5% (7/127) |
Absence of urination ≥ 8 hours postoperatively [1, 12, 13] | 52% | 7,9% (10/127) |
Absence of urination ≥ 12 hours postoperatively [1, 14] | 36% | 6,3% (8/127) |
The first mobilization of patients with POUR was at 13.1±5.0 hours after surgery compared to 9.7±4.4 hours (Student's t = 1.97, p = 0.05) for non-POUR patients.
The secondary outcome parameters of the study involved testing a number of previously reported risk factors to determine whether their presence perioperatively would be associated with POUR. From the preoperative patient-related parameters, none were confirmed as a risk factor for POUR: DM, higher anesthetic risk (ASA III versus ASA I-II), ≥ 3 deliveries in women, female gender, chronic beta-blocker medication, Class II NYHA HF, hypothyroidism, age ≥ 60 years or age ≥ 55 years, previous abdominal surgery, myocardial infarction in patient’s history, cerebrovascular accident (CVA) in medical history.
Among the patient-related preoperative risk factors that are unmodifiable, essential hypertension (HTN) was detected as an independent risk factor for POUR (Table 3).
Table 3. Preoperative risk factors for acute postoperative urinary retention | ||||||
Risk factors | Fisher exact | X2 | p* | Odds ratio | X2 Yates correction | p* |
Diabetes mellitus (DM) | 0.114 | 3.712 | 0.054 | 4.93 | 1.526 | 0.217 |
≥ 3 childbirths | 0.305 | 1.731 | 0.188 | 0.23 | 0.623 | 0.429 |
Anesthetic risk ASA III | 0.680 | 0.380 | 0.538 | 1.62 | 0.040 | 0.842 |
0.205 | 2.258 | 0.132 | 3.11 | 1.175 | 0.278 | |
0.111 | 3.189 | 0.074 | 4.17 | 1.926 | 0.165 | |
BMI > 25 | 1.000 | 0.226 | 0.634 | 1.50 | 0.005 | 0.942 |
Female gender | 0.425 | 1.180 | 0.277 | 2.30 | 0.147 | 0.493 |
HTN | 0.041 | 5.655 | 0.017 | 9.00 | 3.939 | 0.047 |
Beta-blocker medication | 0.425 | 1.180 | 0.277 | 2.30 | 0.470 | 0.493 |
NYHA II HF | 0.426 | 0.591 | 0.441 | 1.77 | 0.118 | 0.730 |
Hypothyroidism | 0.151 | 2.710 | 0.996 | 3.96 | 1.010 | 0.315 |
Association HTN+DM | 0.026 | 10.786 | 0.001 | 23.0 | 5.354 | 0.020 |
Previous abdominal surgery | 0.248 | 1.852 | 0.173 | 3.06 | 0.947 | 0.330 |
Previous myocardial infarction | 0.539 | 0.132 | 0.716 | 1.50 | 0.077 | 0.781 |
Previous CVA | 0.250 | 2.098 | 0.148 | 4.83 | 0.201 | 0.654 |
Note: DM - diabetes mellitus, BMI - body mass index, ASA - American Society of Anesthesiology, CVA – cerebrovascular accident/ stroke, HF II NYHA - NYHA heart failure grade II, HTN – arterial hypertension; *p < 0.05 statistically significant. | ||||||
At the same time, DM, a risk factor often reported in the literature, was parametrically at the limit of statistical significance. This risk factor was further analyzed by applying the extended POUR definition (using the criterion of „lack of micturition in the first 8 hours postoperatively” [12, 13]), proving to be a statistically significant risk factor (OR = 5.1; X2 (1, N = 127) = 5.36, p = 0.021). Returning to the study group where only the need for bladder catheterization was considered as POUR, we analyzed the association between HTN and DM, in our study they were detected as a combination of risk factors for POUR. Similarly, stroke was tested as a risk factor on the group that included both patients with POUR (7/127) and those who had no voiding in the first 8 hours postoperatively (3/127) with confirmation of risk factor status (OR = 4.83 (X2 (1, N = 127) = 7.405, p = 0.006).
From the intraoperative and intra-anesthetic parameters tested for the quality of risk factors for POUR none was confirmed (Table 4).
Table 4. Intraoperative and intra-anesthetic risk factors for acute postoperative urinary retention | ||||||
Risk factors | Fisher exact | X2 | p* | Odds Ratio | X2 Yates correction | p* |
Fasting for fluids ≤ 6 hours | 0.706 | 0.291 | 0.589 | 1.52 | 0.023 | 0.880 |
Surgery duration > 90 min. | 1.000 | 0.016 | 0.899 | 1.11 | 0.087 | 0.767 |
Spinal anesthesia | 1.000 | 0.026 | 0.872 | 1.15 | 0.080 | 0.777 |
Fentanyl ≥ 0,5 mg total | 1.000 | 0.022 | 0.881 | 0.87 | 0.100 | 0.752 |
Atropine administration | 0.103 | 3.564 | 0.059 | 4.48 | 2.211 | 0.137 |
Fentanyl ≥ 0,5 mg + 0,1 mg p/o | 1.000 | 0.156 | 0.693 | 1.78 | 0.134 | 0.714 |
Anesthesia ≥ 95 min. | 1.000 | 0.045 | 0.832 | 0.83 | 0.056 | 0.813 |
Abdomen + lower limb | 1.000 | 0.073 | 0.787 | 1.35 | 0.055 | 0.815 |
Without NSAIDs | 0.129 | 2.801 | 0.094 | 5.88 | 1.297 | 0.255 |
Opioid vs. non-opioid analgesia | 0.678 | 0.070 | 0.792 | 0.80 | 0.036 | 0.850 |
Fluids ≥ 1500 ml i/ op | 0.707 | 0.291 | 0.589 | 1.520 | 0.023 | 0.881 |
Bleeding volume ≤ 400 ml | 0.478 | 0.296 | 0.586 | 0.55 | 0.0216 | 0.883 |
Analgesia (promedol + NSAIDs) | 0.330 | 1.471 | 0.225 | 5.17 | 0.071 | 0.789 |
Fentanyl vs. Morphine p/o | 1.0 | 0.037 | 0.847 | 1.27 | 0.173 | 0.677 |
Note: NSAIDs - non-steroidal anti-inflammatory drugs, min - minutes, i/op - intraoperative, p/o - postoperative; *p < 0.05 statistically significant. | ||||||
Discussions
The present study aimed to test the risk factor associated with POUR for a number of perioperative parameters.
The surgical population studied inadvertently included predominantly male subjects (79/127 (62.2%)). However, according to our results, the prevalence of POUR was equivalent in both women (4/7) and men (3/7). Thus, sex remains a controversial risk factor. Some studies report that male sex is more frequently associated with POUR [2, 3], particularly due to specific pathologies such as benign prostatic hypertrophy [6]. Other studies report that the female sex is more susceptible to POUR [8]. Regarding patients' chronic medication, in our study patients with benign prostatic hypertrophy were excluded, as they were chronically medicated with alpha-receptor blockers (e.g., tamsulosin), which could have been an important factor of bias.
Advancing age increases the risk of POUR, with reports that patients aged ≥ 50 years develop POUR 2.4 to 2.8 times more frequently, which is attributed to progressive neuronal degeneration with bladder dysfunction [2-4, 6]. In our study, however older age (≥ 60 years/ ≥ 50 years) did not confirm its status as a risk factor for POUR. The explanation could lie in the fact that we excluded from the outset the anesthetic population with ASA score > III and orthopedic surgery.
Among the patient-related preoperative risk factors that are unmodifiable, HTN has been detected as an independent risk factor for POUR, consistent with previous studies.
Thus, in our study, patients with pre-existing neurological disorders such as stroke and diabetic neuropathy more frequently developed POUR. Pathophysiologically, the association of POUR and DM can be explained by impaired bladder sensation, decreased capacity, and contractility [15]. Similarly, Keita H. [3] confirmed that pre-existing neurological disorders (cerebral palsy, multiple sclerosis, alcoholic or diabetic polyneuropathy, poliomyelitis) as a risk factor for POUR. The same study reports a direct correlation between bladder volume ≥ 270 ml after surgery and POUR. In our study, there was no possibility to estimate bladder volume using ultrasound, but among the whole group of patients, only one patient stopped the intake of clear liquids 2 hours preoperatively, 60 patients did not ingest clear liquids 6 hours preoperatively, 67 respondents abstained more than 6 hours from preoperative fluid intake. In addition, an infusion volume ≥ 1500 ml intra-anesthetic or hemorrhage ≤ 400 ml were not identified as risk factors for POUR. No patient with peripheral nerve block developed POUR.
Although opioids are known to cause urinary retention, this concept was not confirmed in our study. At the same time, opioid-associated POUR phenomenon has been described at fentanyl doses above 1 mg [16]. In our study, regarding the total dose of fentanyl used perianesthetically no statistically significant differences were identified between fentanyl ≥ 0.5 mg + 0.1 mg postoperatively (10/127) versus fentanyl < 0.5 mg + 0.1 mg postoperatively (17/127). In addition, all patients had intra-anesthetic summary dose of fentanyl ≤ 0.8 mg.
Although opioids are known to cause urinary retention, this concept was not confirmed in our study. At the same time, opioid-associated POUR phenomenon has been described at fentanyl doses above 1 mg [16]. In our study, regarding the total dose of fentanyl used perianesthetically no statistically significant differences were identified between fentanyl ≥ 0.5 mg + 0.1 mg postoperatively (10/127) versus fentanyl < 0.5 mg + 0.1 mg postoperatively (17/127). In addition, all patients had intra-anesthetic summary dose of fentanyl ≤ 0.8 mg.
Patients’ postoperative analgesia was also studied. Thus, 24.4% patients received nonopioid analgesia with NSAIDs (31/127) with 2 cases of POUR (2/31), 30.7% patients beneficiated of promedol (39/127) with 2 cases of POUR recorded (2/39), 27.6% fentanyl (35/127) with another 2 cases of POUR (2/35) and 17.3% morphine (22/127) with only 1 case of POUR (1/22). Cases of POUR were observed both in the opioid analgesia group, regardless of the morphine administered, and in the non-opioid analgesia group.
When analyzing risk factors that may be related to surgical treatment per se, it has been reported that the prevalence of POUR varies depending on the type of surgery and the likelihood of autonomic nerve injury (as a result of total mesorectal excision), pelvic nerve injury or reflex increase in internal sphincter tone (caused by pain in patients undergoing anorectal surgery) [17]. In the present study, no statistical differences were detected between the groups of patients who underwent abdominal and lower limb versus thoracic and upper limb surgery.
Previous studies support that increased volumes of intraoperatively administered fluids would be associated with higher prevalence for POUR [2-4]. In the present trial, we did not determine statistically significant differences between study groups in which ≥ 1500 mL infusible fluids versus < 1000 mL was administered intraoperatively. It is noteworthy that none of the patients infused with 500 mldeveloped POUR.
Strengths and potential biases of the study
One of the strengths is the prospective methodology of the study. Also, to standardize data collection, patients were assessed postoperatively by the same investigator.
The given study has some limitations that need to be taken into consideration. One factor of bias would reside in the small sample and single-center study. Also, in the institution where the study took place, orthopedic surgery patients undergoing spinal anesthesia are inserted with urinary catheters from the beginning of surgery. Therefore, this large group of patients was excluded from the study. Also, patients who developed complications, with admission to Intensive Care Unit, were excluded from the study.
Conclusions
The prevalence of POUR in a single-center surgical population from the Republic of Moldova varies between 5.5% and 7.9%, depending on which criteria for POUR we apply. In this regard, a consensus on diagnostic criteria for POUR is needed.
Patients with HTN and DM developed more frequently POUR. Patients with pre-existing neurological disorders such as positive history for stroke and diabetic polyneuropathy are more susceptible to POUR. It was also determined that the first mobilization of patients with POUR was later compared to patients who did not develop this complication.
Competing interests
None declared.
Authors' contribution
NB developed the study concept, literature review and survey questionnaire, analyzed the collected data, wrote, translated into English and approved the final version of the manuscript. CL recruited the eligible patients, completed the study questionnaire, monitored the patients in the first 24 hours postoperatively, numerated the data in Excel, and contributed to the literature review. Both authors approved the final version of the manuscript.
Informed consent for publication
Obtained.
Ethics approval
Not needed for this study.
Funding
The study had no external funding.
Authors’ ORCID IDs
Natalia Belîi – https://orcid.org/0000-0002-2351-0279
Cătălina Lozan – https://orcid.org/0009-0004-3554-0514
References
Brouwer TA, Rosier PF, Moons KG, et al. Postoperative bladder catheterization based on individual bladder capacity: a randomized trial. Anesthesiology. 2015;122(1):46-54. doi: 10.1097/ALN.0000000000000507.
Baldini G, Bagry H, Aprikian A, et al. Postoperative urinary retention: anesthetic and perioperative considerations. Anesthesiology. 2009;110(5):1139-1157. doi: 10.1097/ALN.0b013e31819f7aea.
Keita H, Diouf E, Tubach F, et al. Predictive factors of early postoperative urinary retention in the postanesthesia care unit. Anesth Analg. 2005;101(2):592-596. doi: 10.1213/01.ANE.0000159165.90094.40.
Agrawal K, Majhi S, Garg R. Post-operative urinary retention: review of literature. World J Anesthesiol. 2019;8(1):1-12. doi: 10.5313/wja.v8.i1.1.
Elsamra SE, Ellsworth P. Effects of analgesic and anesthetic medications on lower urinary tract function. Urol Nurs. 2012;32(6):60-67. doi: 10.7257/1053 816X.2012.32.2.60.
Petros JG, Rimm EB, Robillard RJ, Argy O. Factors influencing postoperative urinary retention in patients undergoing elective inguinal herniorrhaphy. Am J Surg. 1991;161(4):431-433. doi: 10.1016/0002-9610(91)91105-R.
Myles PS, Hunt JO, Moloney JT. Postoperative ‘minor’ complications. Comparison between men and women. Anaesthesia. 1997; 52(4):300-306. doi:10.1111/j.1365-2044.1997.89-az0091.x.
Toyonaga T, Matsushima M, Sogawa N, Jiang SF, Matsumura N, Shimojima Y, Tanaka Y, Suzuki K, Masuda J, Tanaka M. Postoperative urinary retention after surgery for benign anorectal disease: potential risk factors and strategy for prevention. Int J Colorectal Dis. 2006;21(7):676-682. doi: 10.1007/s00384-005-0077-29.
Changchien CR, Yeh CY, Huang ST, Hsieh ML, Chen JS, Tang R. Postoperative urinary retention after primary colorectal cancer resection via laparotomy: a prospective study of 2,355 consecutive patients. Dis Colon Rectum. 2007;50(10):1688-1696. doi: 10.1007/s10350-007-0305-7.
Choi S, Awad I. Maintaining micturition in the perioperative period: strategies to avoid urinary retention. Curr Opin Anaesthesiol. 2013;26(3):361-7. doi: 10.1097/ACO.0b013e32835fc8ba.
Ozturk NK, Kavakli AS. Use of bladder volume measurement assessed with ultrasound to predict postoperative urinary retention. North Clin Istanb. 2017;3(3):209-216. doi: 10.14744/nci.2016.03164.
Cataldo PA, Senagore AJ. Does alpha sympathetic blockade prevent urinary retention following anorectal surgery? Dis Colon Rectum. 1991;34(12):1113-1116. doi: 10.1007/BF02050073.
Paulsen EK, Porter MG, Helmer SD, Linhardt PW, Kliewer ML. Thoracic epidural versus patient-controlled analgesia in elective bowel resections. Am J Surg. 2001;182(6):570-577. doi: 10.1016/S0002-9610(01)00792-9.
Dobbs SP, Jackson SR, Wilson AM, Maplethorpe RP, Hammond RH. A prospective, randomized trial comparing continuous bladder drainage with catheterization at abdominal hysterectomy. Br J Urol. 1997;80(4):554-556. doi: 10.1046/j.1464-410X.1997.t01-1-00376.x.
Kebapci N, Yenilmez A, Efe B, Entok E, Demirustu C. Bladder dysfunction in type 2 diabetic patients. Neurourol Urodyn. 2007;26(6):814-9. doi: 10.1002/nau.20422.
Lamonerie L, Marret E, Deleuze A, Lembert N, Dupont M, Bonnet F. Prevalence of postoperative bladder distension and urinary retention detected by ultrasound measurement. Br J Anaesth. 2004;92(4):544-546. doi: 10.1093/bja/aeh099.
Jang HJ, Kang S, Lee S, Park JS, Kim D, Ahn S. Randomized controlled trial of tamsulosin for prevention of acute voiding difficulty after rectal cancer surgery. World J Surg. 2012;36(11):2730-7. doi: 10.1007/s00268-012-1712-z.