Introduction
Prematurity remains one of the most important health problems worldwide, which is associated with high morbidity and mortality, high costs of medical care and increased risk of disability. Bronchopulmonary dysplasia (BPD) is considered to be one of the forms of chronic lung damage in newborns, with predilection of preterm infants, which frequently develops as a complication of artificial pulmonary ventilation (APV) [1-3]. It evolves primarily with the primary involvement of the bronchioles and lung parenchyma, with onset of emphysema, fibrosis and/or impairment of alveolar replication and characterized by specificity of radiological changes in the first months of life and regression of clinical and imaging manifestations as the child grows [4-6].
Data on BPD frequency vary considerably worldwide, and the incidence rate is influenced by the used diagnostic criteria ("28 days" or "36 weeks, oxygen dependence"), the mortality of premature newborns, the studied population, the level of technical equipment in neonatal, pulmonology, intensive care units, etc. On average, according to literature data, BPD develops in 30% of newborns who require mechanical ventilation, and about 40% of infants with extremely low birth weight (birth weight of <1000 g) develop DBP [7]. Unlike other causes of morbidity that complicate severe prematurity, the incidence of DBP has not decreased over the past few years [8, 9].
BPD remains a primary cause of chronic respiratory pathology despite advances in neonatal medicine. It is also recognized as a distinct nosological entity among surviving preterm infants, with a frequency among living preemies of 20-40%. Worldwide, it has been estimated that more than 15 million babies are born prematurely. Newborns with severe degrees of prematurity, especially those with BPD, frequently develop respiratory symptoms (usually coughing and wheezing) as well as frequent hospitalizations in the first years of life. Moreover, this clinical entity is associated with increased respiratory morbidity and mortality [9], in particular, during the first two years of life, due to the persistence of respiratory symptoms and the higher number of hospitalizations compared to preterm infants without BPD [10].
In recent years progress has been made in pediatric thoracic imaging, enhancing the diagnostic potential of BPD, facilitating treatment guidance for respiratory distress syndrome (RDS), reducing the likelihood of further progress of BPD and long-term follow-up of chronic pulmonary pathology [1].
First-line radiological examinations, such as pulmonary radiography, are useful in determining the severity of BPD, differentiating it from atelectasis, pneumonia, and air loss syndrome, with changes such as decreased lung volumes, areas of atelectasis and hyperinflation, pulmonary edema and pulmonary interstitial emphysema being noted. The findings on radiological examination range from signs of pulmonary hyperinflation with thickening of the bronchi and atelectasis, to the presence of radiopaque images of pulmonary fibrosis, large airborne cysts, and interstitial emphysema. The trunk of the pulmonary artery may be highlighted due to associated pulmonary hypertension, and in extremely severe cases, cardiomegaly may also be noted [11].
Nevertheless, the recognized major radiological criteria include significant thoracic distension predominantly in the basal areas, opacities with blurred contour, poorly delineated in the middle and upper regions of the lungs, subsegmental atelectasis, fibrotic opaque sectors, areas of emphysema, linear or round opacities [12, 13].
The aim of the research was to evaluate chest x-rays in children with bronchopulmonary dysplasia.
Material and methods
This study represents an analysis of a cohort of 105 premature babies admitted to the Clinic of Pulmonology of the Institute of Mother and Child with a positive history of premature birth and with RDS supported during the neonatal period. Two groups were created: the study group of 53 children (33 boys and 20 girls) with the diagnosis of bronchopulmonary dysplasia and the control group of 52 children (27 boys and 25 girls) who did not develop BPD. The diagnosis of BPD was established based on the analysis of perinatal, history, clinical, laboratory and instrumental data, especially pulmonary imaging, based on international criteria [14]. Radiological examination was performed on all children included in the study. The study complied with the international standards of medical ethics, developed by the Declaration of Helsinki, regarding confidentiality and personal data protection of the participants. The research was approved by the Research Ethics Committee of Nicolae Testemiţanu State University of Medicine and Pharmacy (minutes № 73 from 31.05.2017). The resulting data were disclosed only to the concerned participant; the personal data of each subject were not used and will not be used for any other purpose. The data collected from the primary sources were introduced in the electronic database, whereas the statistical processing was performed using the SPSS (Statistical Package for the Social Sciences) version 20.
Results
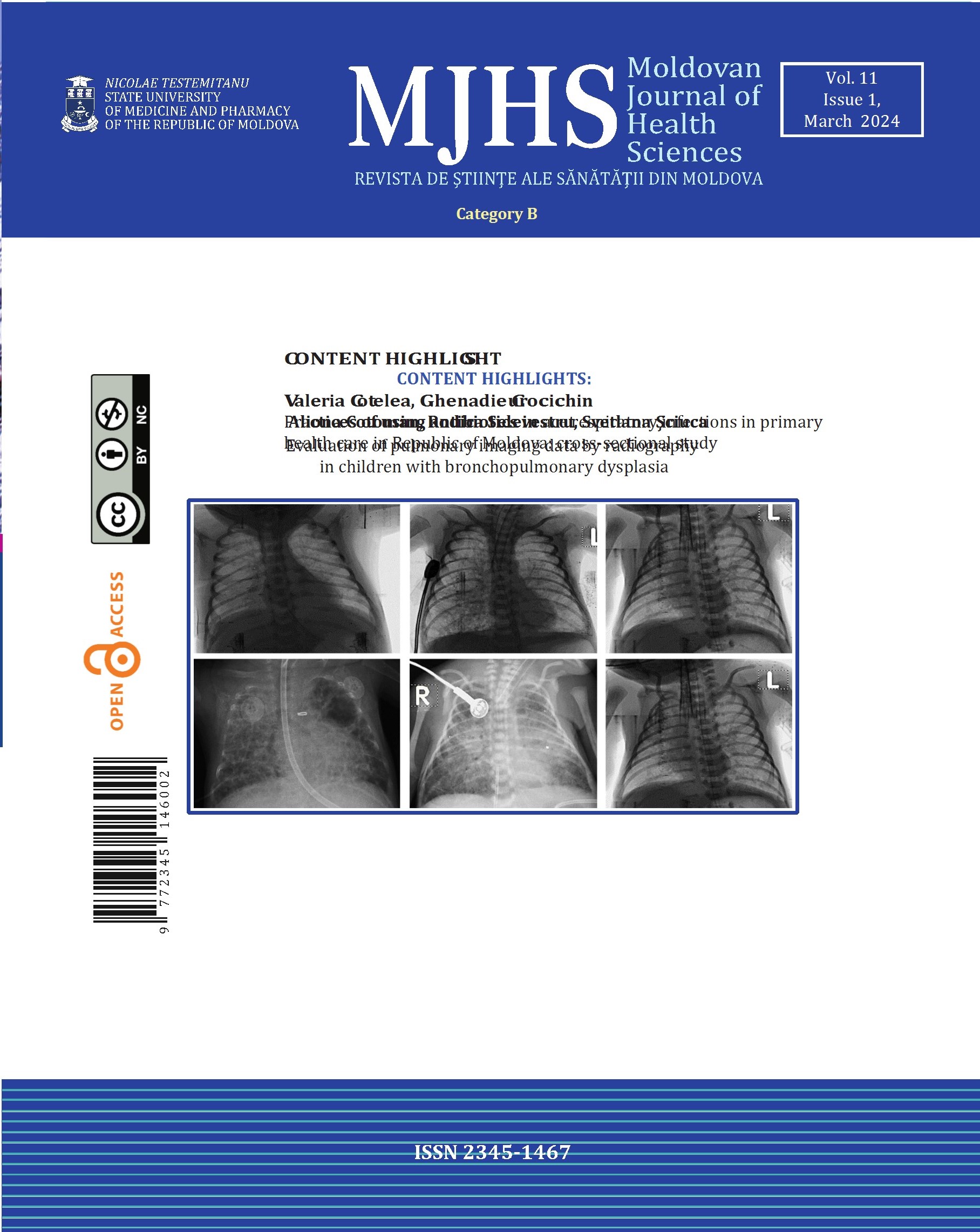
Pulmonary radiography was performed on all children included in the research. Thus, the children in the study (Fig. 1) had predominantly radiological changes of atelectasis type, with discoidal changes detected radiologically in 49.1% (n=26) of children with BPD and in 13.5% (n=7) of children in the control group (χ2=15.431; p<0.0001). Subsegmental changes were reported in 47.2% of children with BPD (n=25) and in 25.0% of children without BPD (n=13) (χ2=5.586; p=0.018). Areas of pulmonary emphysema in the study group were noted in 62.3% (n=33), while in the control group, they were observed in only 5.8% (n=3) (χ2=37.182; p<0.0001). Opaque fibrosis sectors in children with BPD were identified in 50.9% (n=27 cases), and in children without BPD in 11.5% (n=6) (χ2=18.911; p<0.0001). Signs of pulmonary hypertransparency were observed in 47.2% of children with BPD (n=25) compared to single cases (1.9%) in control subjects (χ2=28.843; p<0.0001). Microcystic formations were recorded with a higher share in 41.5% of children with BPD (control group – 5.8% cases) (χ2=18.482; p<0.0001).
The periods of hospitalization resulted in radiological changes with pneumonic foci character (Fig. 1) in 52.8% (n=28) among those in the first group, and in those without BPD in 73.1% (n=38) (χ2=4.609; p=0.032), and bronchoobstructive syndrome was noted in children with BPD in 58.5% (n=31), vs 28.8% (n=15) in the control group (χ2=9.370; p=0.002).
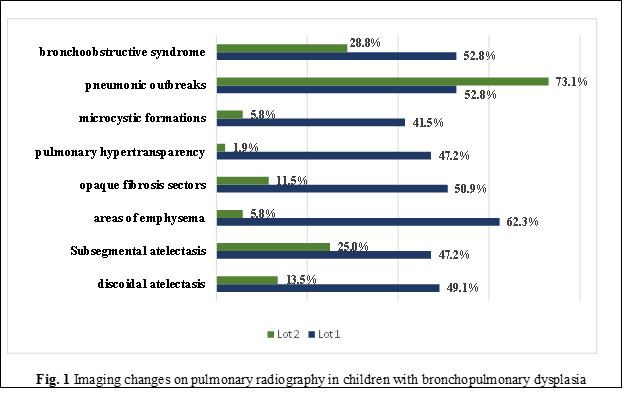
The analysis of radiology results in relation to the severity degrees of BPD (Table 1) noted changes such as discoidal atelectasis (Fig. 2f), present in 50% of those with mild grade (present in every second child, n=12), 38.5% with medium grade (n=5), and 56.3% with severe grade (n=9) (χ2=16.502; p<0.001). Subsegmental atelectasis (Fig. 2b), among those with mild BPD, was recorded in 41.7% (n=10) and in 38.5% with medium grade (n=5) (χ2=7.956; p=0.047). Areas of pulmonary emphysema in children with mild BPD were detected in every second case – 58.3% (n=14), in every third child with average severity (38.5%, n=5), and in most of those with severe grade (87.5%, n=14) (χ2=45.138; p<0.0001).
Microcystic formations (Fig. 2d, e), which are often characteristic of pulmonary diseases with obstructive genesis, were present in children with mild BPD degree in 33.3% (n=8), in medium severity – 53.8% (n=7), and in severe grade – 43.8% (n=7) (χ2=20.502; p<0.0001).
Pneumonic foci were also visualized (Fig. 2. a, c, f): in children with mild BPD in 41.7% cases (n=10), in those with medium grade – in 76.9% cases (n=10), and in every second child with severe BPD (n=8) (χ2=9.177; p=0.027).
At the same time, in pediatric subjects with BPD in pulmonary imaging evaluation, bronchoobstructive syndrome was also present (Fig. 2 c, e, f), noted in 62.5% (n=15) in those with mild degree, 5% (n=5) in medium grade, and 68.8% (n=15) in severe grade (χ2=12.329; p=0.006). The imaging expression of broncho-obstructive disorders is also relevant through signs of pulmonary hypertransparency, which in children with mild BPD was found in 45.8% (n=11), in the middle degree – 38.5% (n=5), and in severe degree – 56.3% (n=9), (χ2=30.103; p<0.0001).
Foci of pulmonary fibrosis are mentioned in the literature as imaging signs suggestive of BPD and were recorded in the research performed. Opaque fibrosis sectors (Fig. 5, 6, 7) were identified in 54.2% (n=13) of subjects with a mild degree of BPD, in 46.2 (n=6) of those with medium severity, and in 50.0% (n=8) of those with severe degree (χ2=19.172; p<0.0001).
Table 1. Lung imaging changes in varying degrees of severity of BPD | |||||||
Lung imaging signs | BPD light grade (n=24) | BPD medium grade (n=13) | BPD severe grade (n=16) |
p | |||
Abs | % | Abs | % | Abs | % |
| |
Discoidal atelectasis | 12 | 50,0 | 5 | 38,5 | 9 | 56,3 | 0,001 |
Subsegmental atelectasis | 10 | 41,7 | 5 | 38,5 | 10 | 62,5 | 0,047 |
Areas of emphysema | 14 | 58,3 | 5 | 38,5 | 14 | 87,5 | <0.0001 |
Microcystic formations | 8 | 33.3 | 7 | 53,8 | 7 | 43,7 | <0.0001 |
Pneumonic outbreaks | 10 | 41,7 | 10 | 76,9 | 8 | 50,0 | 0,027 |
Broncho-obstructive syndrome | 15 | 62,5 | 5 | 38,5 | 11 | 68,8 | 0,006 |
Opaque fibrosis sectors | 13 | 54,2 | 6 | 46,2 | 8 | 50,0 | <0.0001 |
Pulmonary hypertransparency | 11 | 48,5 | 5 | 38,5 | 9 | 56,3 | <0.0001 |
| Note: BPD- bronchopulmonary dysplasia, ABS- absent, n – number, % - percent | |||||||
For the objectification of imaging data characteristic of the evolutionary development of BPD, radiographic images with various modifications are presented (Fig. 2 a-f).
Discussion
BPD is a chronic lung disease involving damage to the alveoli and pulmonary vessels due to neonatal lung underdevelopment, oxygen therapy used in the postnatal period, and associated infections [15], which causes delayed lung development, further contributing to insufficient development of the alveoli and pulmonary capillaries. BPD was first described as a chronic lung disease associated with RDS by Northway in 1967.
Clinically, BPD is established as a diagnosis if the newborn requires supplemental oxygen at 28 days after birth and has specific features on chest radiograph at 36 weeks' corrected gestational age. Depending on the characteristics of the lung x-ray images, it is divided into 4 stages, respectively: 1st stage – similar findings in chest x-ray with RDS (1–3 days), 2nd stage – bilateral opacification (4–10 days), 3rd stage – density uneven bilateral lung fields, with bands or irregular shadows (11–30 days), 4th stage – bilateral opacities with lung hyperinflation, atelectasis (30 days).
The survival of extremely premature infants and very low birth weight preterm infants has steadily improved thanks to the use of lung surfactant and various protective ventilation techniques [16]. Approximately 42% of newborns weighing less than 750 g and only 4% of newborns weighing between 1251 and 1500 g develop BPD.
According to a study conducted in China, the incidence of BPD was about 1.28% among preterm infants compared to 19.3% among extremely premature babies (<28 weeks gestation, in 2011) [17, 18]. Factors influencing the severity of BPD among premature babies were also analyzed, demonstrating that birth weight and gestational age correlated with both the incidence and the severity of BPD. Additionally, low birth weight (<1000 g) and low gestational age (<28 weeks) were associated with a higher incidence and severity of BPD [6, 14].
BPD associated with obstructive ventilation impairment and pulmonary emphysema as an end result is recognized as a new type of BPD, which is significantly different from the typical form in terms of clinical and pathological characteristics and outcomes. Consequently, the conventional 4-step radiographic classification for the typical form cannot be applied to the new type.
In the study, chest X-ray images were compared between mild, moderate, and severe forms of BPD, revealing changes such as discoidal atelectasis, areas of emphysema, opaque fibrosis sectors, pulmonary hypertransparency, and microcystic formations, albeit with varying degrees of prominence. Although chest X-ray is not a definitive tool for assessing the severity of BPD, it still holds a certain predictive value; specifically, the more severe the BPD, the sooner characteristic changes in chest X-ray will manifest. However, it should be noted that chest radiography has somewhat low diagnostic specificity for BPD in children. Therefore, it is used as an auxiliary tool for the diagnosis and staging of this pathology, considering its limitations.
Conclusions
Pulmonary radiography is one of the diagnostic methods for BPD in premature infants and can record discoidal atelectasis, areas of emphysema, opaque sectors of fibrosis, pulmonary hypertransparency, and microcystic formations. The research confirms the importance of evaluating the type and extent of radiological changes in premature infants based on the severity of BPD. It has been demonstrated that this method is effective both in practical clinical settings and scientific work.
Competing interests
None declared.
Patient consent
Obtained.
Ethics approval
The research was approved by the Research Ethics Committee of Nicolae Testemitanu State University of Medicine and Pharmacy (report no. 73 of 31.05.2017).
Authors’ contribution
The authors contributed equally to the research of the scientific literature, the selection of the bibliography, the reading and analysis of biographical references, the writing of the manuscript and its peer review. All authors have read and approved the final version of the article.
Acknowledgements and funding
Nothing to declare.
Authors’ ORCID IDs
Aliona Cotoman – https://orcid.org/0009-0009-9729-6912
Rodica Selevestru – https://orcid.org/0000-0002-8923-3075
Svetlana Şciuca – https://orcid.org/0000-0003-1091-9419
References
Gien J, Kinsella JP. Pathogenesis and treatment of bronchopulmonary dysplasia. Curr Opin Pediatr. 2011 Jun;23(3):305-13. doi: 10.1097/MOP.0b013e328346577f.
Jensen EA, Schmidt B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol. 2014 Mar;100(3):145-57. doi: 10.1002/bdra.23235.
Şciuca S, Curteanu A, Selevestru R, Cotoman A, Ceahlău M; Ministry of Health, Labor and Protection of the Republic of Moldova. Bronchopulmonary dysplasia in children: National clinical protocol (NCP-393). Chisinau: The Ministry; 2021. 49 p. Romanian.
Soll RF, Edwards EM, Badger GJ, et al. Obstetric and neonatal care practices for infants 501 to 1500 g from 2000 to 2009. Pediatrics. 2013;35(1):273-281. doi: 10.1542/peds.2013-0501.
Șciuca S. [Essentials in child pulmonology]. Chisinau; 2007. 255 p. Romanian.
Ceahlău M, Selevestru R, Cotoman A, Şciuca S. Predictors of bronchopulmonary dysplasia in premature babies. Bull Acad Sci Mold. Med Sci. 2020;(2/66):132-135.
Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015 Sep 8;314(10):1039-51. doi: 10.1001/jama.2015.10244.
Horbar JD, Carpenter JH, Badger GJ, Kenny MJ, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. 2012;129(6):1019-1026. http://dx.doi.org/10.1542/peds.2011-3028.
Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116(6):1353-1360. https://doi.org/10.1542/peds.2005-0249.
Doyle LW, Faber B, Callanan C, et al. Bronchopulmonary dysplasia in very low birth weight subjects and lung function in late adolescence. Pediatrics. 2006;118(1):108-13. doi: 10.1542/peds.2005-2522.
Czarnecki ŁM. Assessment of chest X-ray images in newborns with respiratory disorders. Kardiochir Torakochirurgia Pol. 2015 Mar;12(1):83-86. doi: 10.5114/kitp.2015.50578.
Semple T, Akhtar MR, Owens CM. Imaging bronchopulmonary dysplasia - a multimodality update. Front Med (Lausanne). 2017 Jun 29;4:88. doi: 10.3389/fmed.2017.00088.
Cotoman A, Ceahlău M, Selevestru R, Crivceanschii E, Şciuca S. Bronchopulmonary dysplasia in children in tomographic images. Mold J Health Sci. 2022;29(3 Suppl):395.
Şciuca S, Ceahlău M, Cotoman A, Selevestru R. Clinical evaluation of the severity of bronchopulmonary dysplasia in children from premature births. Bull Acad Sci Mold. Med Sci. 2023;(3/77):127-132.
Parat S, Mhanna MJ. Respiratory management of extremely low birthweight infants: survey of neonatal specialists. World J Pediatr. 2016 Aug;12(3):314-319. doi: 10.1007/s12519-016-0024-z.
Stroustrup A, Trasande L. Epidemiological characteristics and resource use in neonates with bronchopulmonary dysplasia: 1993-2006. Pediatrics. 2010 Aug;126(2):291-297. doi: 10.1542/peds.2009-3456.
Chang LW. Incidence and risk factors of bronchopulmonarydysplasia in premature infants in 10 hospitals in China. Zhonghua Er Ke Za Zhi. 2011;49(9):655-62.
Li Y, Wei QF, Pan XN, et al. Influencing factors for severity of bronchopulmonary dysplasia in preterm infants. Zhongguo Dang Dai Er Ke Za Zhi. 2014;16(10):1014-8.