Introduction
The Novel Coronavirus Disease (COVID-19) is an infectious illness that has a pandemic spread since December 2019, infecting over 543,200,000 of the world’s population with a 1% of current mortality rate [1].
From the total number of cases, those with asymptomatic, mild, and moderate manifestations represent approximately 80% and the rest of them get severe and critical forms. The rate of Intensive Care Unit (ICU) admission of COVID-19 patients is 11% of the total number of confirmed cases [2]. In patients who have severe and critical manifestation predominates the phenotype of a systemic inflammation, which leads to damage of target organs with tropism for SARS-COV-2 virus (lungs, heart, arterial vascular system, kidneys, ileum and bladder) and The Multiple Organ Dysfunction Syndrome [3, 4].
The Risk factors for severe evolution of COVID-19 are: comorbidities, age older than 65 years, low lymphocytes number, high neutrophil-lymphocyte ratio, high level of D-Dimers, urea, C-Reactive Protein (CRP), ALT, AST and procalcitonin, as well as low PaO2/FiO2 ratio and platelets count etc. [5].
Of the total number of hospitalized COVID-19 non-ICU patients, 33% develop acute respiratory distress syndrome (ARDS), and 26% of them require transfer to ICU. From the total number of COVID-19 ICU-hospitalized patients, 63% are on mechanical ventilation (MV) and approx. 75% are confirmed with ARDS with a mortality rate of up to 93% [6]. This high incidence of ARDS and mortality rate make the pulmonary manifestation of COVID-19 the greatest therapeutic and respiratory support challenge.
The average rate of non-invasive ventilation (NIV) used as respiratory support in COVID-19 is 25.5% [7]. Unfortunately, the predictors of NIV failure as well as clear indications of MV remain debatable. In this context, it is very important to highlight the factors that correlate with NIV failure and would predict the optimal timing for conversion to MV.
Materials and methods
Study population. Was performed the retrospective analysis of COVID-19 patients with acute respiratory failure admitted to the ICU of the Institute of Emergency Medicine, Chisinau, Republic of Moldova, between July 2020 and October 2020 who were connected to NIV. The Research Ethics Committee of Nicolae Testemiţanu State University of Medicine approved the study and Pharmacy of the Republic of Moldova (minutes No.4 from 07.07.2021).
The inclusion study criteria was need for non-invasive ventilation (BiPAP or PSV) with a duration of more than 24 hours from the initiation. NIV was used more than 20h out of 24h and with application of facemask Criteria for non-invasive ventilation were lack of response to conventional oxygen therapy, absence of tachypnea more than 30-35 respiration/min, absence of severe acidosis or hypercapnia, cooperative patient. Patients in whom non-invasive ventilation was used as a post-extubation support method, or CPAP mode, or were connected to mechanical ventilation in less than 24h after NIV initiation, were excluded from the study. Eligible patients were divided in 2 groups: NIV success – patients who were weaned from non-invasive support with respiratory improvement and NIV failure – patients who were connected to mechanical ventilation after more than 24h of non-invasive ventilation. All patients received standard treatment according to the institutional protocol (corticosteroids (methylprednisolone 1mg/kg/day), vitamin therapy, anticoagulants (LMWH or intravenous unfractionated heparin), and antibiotic therapy if necessary. The intubation criteria were based on local institutional practice, including disorder of consciousness, respiratory decompensation (respiratory rate > 30- 35 r/ min, participation of auxiliary muscles in the respiratory act) and severe hypoxemia (SpO2 < 85% on maximal non-invasive support).
Data collection. All information was collected from the SiaamS Electronic Medical Record database used in the Institute of Emergency Medicine, Chisinau.
The study was based on the analysis of the following parameters:
Demographic: age, sex, comorbidities (hypertension, diabetes mellitus, obesity), ISARIC (International Acute Respiratory Infection Consortium) score at admission in ICU;
Laboratory: neutrophil-lymphocyte ratio (N/L ratio), lymphocytes count, platelet count, WBC count, urea, creatinine, CRP, D-Dimers level. All parameters were evaluated at admission, at the initiation of NIV, at 24h-48h of NIV and at 72-96h of NIV. In case of multiple samples extraction in these periods, the worse values of these parameters were selected.
Respiratory: the ratio of pulse oximetry/fraction of inspired oxygen to respiratory rate (ROX index) was evaluated at the initiation of NIV and then every 12 hours up to 76 hours of NIV.
Outcomes: There were considered as outcomes the duration of NIV, association of delirium (according to the DSM5 criteria) [8], need for sedation, ICU and hospital length of stay, NIV success or failure, survival.
Statistical analysis. For continuous variables, the established confidence interval was 95%, all other data has been presented as percentage, median and interquartile range. Category variables were reported as number or percentage. Because of non-parametric distribution, The Kruskal-Wallis H test was used for continuous variables and the Fisher’s exact test or chi-squared test was used for category data.
The diagnostic predictive ability was calculated by statistical analysis of receiver operating curves (ROC). Statistical significance was assigned to the data with a p <0.05. SPSS version 26.0 was used to analyze the data (IBM Corp, Armonk, NY, USA).
Results
A total of 482 patients with severe or critical form of COVID-19 were admitted to ICU, and 154 were enrolled (Figure 1). Demographic and clinical data of the patients are reported in Table 1.
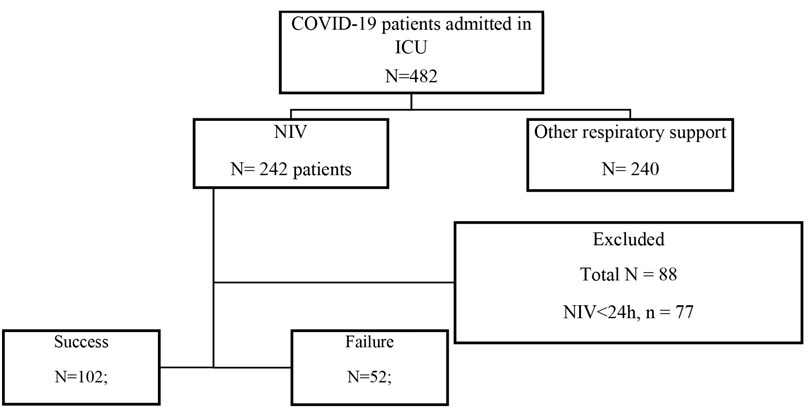
Fig. 1 Study flow chart. ICU - Intensive Care Unit; NIV - non-invasive ventilation; ID - incomplete data.
| Variable | NIV success group (n=102) (66%), Median (IQR) | NIV failure group (n=52) (34%) | ρ value |
|---|---|---|---|
| Age, years | 61.5 (54-69.25) | 67.5 (60.25-73) | 0.02 |
| Male, n (%) | 43 (42%) | 27 (52 %) | 0.25 |
| Hypertension, n (%) | 75 (74%) | 46 (88%) | 0.033 |
| Diabetes mellitus, n (%) | 35 (34%) | 16 (31%) | 0.65 |
| Obesity, n (%) | 36 (35%) | 21 (40%) | 0.53 |
| Day of illness at admission | 7 (6-9) | 7 (4-9.25) | 0.086 |
| ISARIC mortality score points | 10 (8-12) | 12 (10-14) | 0.001 |
| ISARIC mortality score, % | 23 (14-33) | 33 (23-45) | 0.001 |
| ISARIC deterioration score points | 615 (544.5-680.5) | 673.5 (580-802.5) | 0.03 |
| ISARIC deterioration score, % | 69 (57.75-78) | 77 (63.75-89) | 0.03 |
| Outcome | |||
| NIV duration, days | 5 (4-7) | 6 (4-9) | 0.46 |
| Delirium, n (%) | 20 (20%) | 31 (60%) | <0.001 |
| Need for sedation, n (%) | 49 (48%) | 43 (83%) | <0.001 |
| ICU stay, days | 7 (6-10) | 14 (10-17) | <0.001 |
| Hospitalization, days | 16.5 (13-23) | 14 (10-18.75) | <0.001 |
| In-hospital mortality, n (%) | 3 (0.3%) | 50 (96%) | <0.001 |
Note: ISARIC: International Severe Acute Respiratory Infection Consortium score; ICU - Intensive Care Unit; NIV - non-invasive ventilation. Data are presented as median (interquartile range IQR)
Patients in the NIV success group were younger: 61.5 (IQR 54-69.25) vs 67.5 (IQR 60.25-73), (p=0.02). The percentage of male patients was 52% in the NIV failure group vs 42% in the NIV success group (p=0.25). The rate of hypertension was higher in patients who failed the NIV: 88% vs 74% (p=0.033), which represents the risk for NIV failure, OR = 1.203 (CI 95% 1.033-1.401, p=0.0033). The incidence of diabetes mellitus and obesity did not register significant differences between groups. The ISARIC score for deterioration and mortality measured at ICU admission was higher in patients with NIV failure: ISARIC Deterioration score (points/%): 673.5 (77%) vs 615 (69%), (p=0.03) and ISARIC Mortality score (points/%): 12 (33%) vs 10 (23 %), (p=0.001). ICU length of stay (days) was twice shorter in NIV success group, but with longer time of hospitalization. Association of delirium was registered in 20% of cases in NIV success group vs 60% in NIV failure group, (p=0.001). Patients in the NIV success group had less need for sedation 48% vs 83%, (p=0.001). The presence of delirium and the need for sedation are related to the risk of NIV failure: OR=3.04 (CI 95%, 1.934-4.779, p=0.001) for delirium and OR=1.721 (CI 95%, 1.358-2.182, p=0.001) for the need for sedation. Only two patients survived in the NIV failure group, corresponding with 96% of mortality in case of failure. In the NIV success group 3 patients died due to documented pulmonary embolism after successfully weaning from non-invasive support and discharge from ICU. The Table 2 presents the laboratory parameters of both groups.
| Variable | NIV success group (n=102) (66%) | NIV failure group (n=52) (34%) | p Value |
|---|---|---|---|
| At admission | |||
| Urea, mmol/l | 6.7 (5.25-8.3) | 8.3 (6.22-11.6) | 0.001 |
| At NIV initiation | |||
| N/L ratio | 9 (5-13) | 10 (6-18.25) | 0.047 |
| Urea, mmol/l | 6.7 (5.45-8.3) | 8.05 (6.37-10.55) | 0.003 |
| 24-48h of NIV | |||
| N/L ratio | 8 (6-15) | 15 (8-24) | 0.001 |
| Leucocytes, 109/l | 9.4 (7.5-11.8) | 11.3 (8.3-14.9) | 0.007 |
| Urea, mmol/l | 6.7 (5.45-8.7) | 8.25 (6.23-10.9) | 0.016 |
| CRP, mg/l | 37.7 (18.5-82.7) | 69.6 (24-126.25) | 0.032 |
| 72-96h of NIV | |||
| N/L ratio | 11 (5-15) | 18 (9-30) | 0.001 |
| Lymphocyte, 109/l | 0.86 (0.5-1.38) | 0.69 (0.36-0.94) | 0.019 |
| Leucocytes, 109/l | 9.2 (7.4-11.5) | 12 (8-14.8) | 0.001 |
| CRP, mg/l | 31.25 (15.32-63.75) | 74 (24-138.5) | 0.026 |
| D-dimer, mg/l | 1.44 (0.58-4.67) | 4.4 (1.67-7.62) | 0.002 |
Note: N/L ratio: neutrophil-lymphocyte ratio; CRP, C-reactive protein; NIV: non-invasive ventilation. Data are presented as median (interquartile range IQR)
The patients with success of NIV had lower levels of urea (mmol/l) during the hospitalization: at admission to 24-48h of non-invasive ventilation. The neutrophil/lymphocyte ratio recorded statistically significant difference between groups from the start of NIV ventilation and during the 96h of NIV. In the group with NIV failure, the value of C - reactive protein (mg/l) was two-fold higher: 69.6 (IQR 24-126.25) vs 37.7 (IQR 18.5-82.7), p=0.032 at 24-48h and 74 (IQR 24-138.2) vs 31.25 (IQR 15.32-63.75) at 72-96h of NIV. The notable difference between the two groups was found in D-dimer (mg/l) values at 72-96h of NIV: 1.44 (IQR 0.58-4.67) (NIV success) vs 4.4 (IQR 1.67-7.62) (NIV failure), p=0.026 and in lymphocytes number (10^9/l): 0.86 (IQR 0.5-1.38) (NIV success) vs 0.69 (IQR 0.36-0.94) (NIV failure), p=0.019. WBC count was higher in the NIV failure group at 24-48h and 72-96h of NIV. It was not registered the statistically significant difference in platelets count values during the non-invasive ventilation. Table 3 shows the relationship between different parameters and risk for NIV failure. The variables that presented the difference in values between the two groups were stratified. Were identified the association between NIV failure and the following parameters: age > 60 years; N/L ratio more than 9.8 at 24-48h and 72-96h of NIV; Leucocytes count > 10x10^9/l at 24-48h, and 72-96h of NIV; Urea > 7.5 mmol/l at admission, NIV initiation and 24h-48h of NIV; D-dimer > 1.5 mg/l at 72-96h of NIV. Values of CRP more than 36 mg/l were correlated with NIV failure only at 72-96h of non-invasive ventilation.
| Variable | OR ( CI 95% ) | p-value |
|---|---|---|
| Hypertension | 1.203 (1.033-1.401) | 0.033 |
| Delirium | 3.04 (1.934-4.779) | 0.0001 |
| Need for sedation | 1.721 (1.358-2.182) | 0.0001 |
| Age, > 60 | 1.436 (1.146-1.799) | 0.004 |
| N/L ratio, > 9.8 at 24-48h of NIV | 1.607 (1.171-2.205) | 0.005 |
| N/L ratio, > 9.8 at 72-96h of NIV | 1.396 (1.082-1.800) | 0.016 |
| Leucocytes, >10x109/l at 24-48h of NIV | 1.545 (1.130-2.114) | 0.009 |
| Leucocytes, >10x109/l at 72-96h of NIV | 1.667 (1.218-2.280) | 0.002 |
| CRP, > 36 mg/l at 24-48h of NIV | 1.242 (0.893-1.727) | 0.213 |
| CRP, >36 mg/l at 72-96h of NIV | 1.512 (1.099-2.082) | 0.018 |
| Urea, >7.5 mmol/l at admission | 1.527 (1.108-2.104) | 0.013 |
| Urea, >7.5 mmol/l at NIV initiation | 1.459 (1.032-2.061) | 0.038 |
| Urea, >7.5 mmol/l at 24-48h of NIV | 1.471 (1.052-2.058) | 0.029 |
| D-dimer, > 1.5 mg/l at 72-96h of NIV | 1.545 (1.093-2.185) | 0.028 |
Note: N/L = neutrophil/lymphocyte ratio; NIV = non-invasive ventilation; CRP = “C” reactive protein.
| Variable | NIV success group (n=102) (66%), Median (IQR) | NIV failure group (n=52) (34%) | p Value |
|---|---|---|---|
| ROX index at NIV initiation | 6.03 (5.5-6.5) | 5.21 (4.57-6.08) | <0.001 |
| ROX index at 12h of NIV | 6.17 (5.65-6.73) | 5.48 (4.89-6.13) | <0.001 |
| ROX index at 24h of NIV | 6.22 (5.77-6.72) | 5.49 (5.01-6.13) | <0.001 |
| ROX index at 36h of NIV | 6.23 (5.81-6.78) | 5.52 (4.86-6.07) | <0.001 |
| ROX index at 48h of NIV | 6.23 (5.9-6.72) | 5.35 (4.85-5.67) | <0.001 |
| ROX index at 60h of NIV | 6.23 (5.9-6.72) | 5.48 (4.95-5.71) | <0.001 |
| ROX index at 72h of NIV | 6.39 (6.02-7.05) | 5.41 (4.88-5.71) | <0.001 |
Note: ROX index: ratio of pulse oximetry/fraction of inspired oxygen to respiratory rate; NIV: non-invasive ventilation. Data are presented as median (interquartile range IQR)
The ROX index (Table 4) values was higher in the NIV success group from NIV initiation until 72h, and ROC curves of its predictive model for NIV failure are in Table 5, demonstrating moderate accuracy at NIV initiation (cut-off value: 5.65) with AUC 0.696 (p=0.001), 70% sensitivity, and 59.6% specificity. ROX index values from 12 to 36h show good prediction accuracy: cut-off 5.68, AUC 0.708 (p=0.001), sensitivity 74.5%, specificity 59.6% at 12h; cut-off 5.86, AUC 0.718 (p=0.001), sensitivity 73.5%, specificity 65.4% at 24h; cut-off 5.68, AUC 0.745 (p=0.001), sensitivity 74.5%, specificity 71.1% at 36h. From 48h to 72h of NIV, ROX index demonstrates a very good predictive model. Its values are: for ROX index at 48h of NIV sensibility 85.3% and specificity 80% at cut-off value 5.71, AUC 0.812, p=0.001, for ROX index at 60h of NIV sensibility 85.3% and specificity 79.2% at cut-off value 5.74, AUC 0.800, p=0.001 and for ROX index at 72h of NIV sensibility 88.1% and specificity 80% at cut-off value 5.74, AUC 0.841, p=0.001 (Figure 2).
| Variable | Sensitivity (%) | Specificity (%) | Cut-Off Value | AUC (95% CI) | p-Value |
|---|---|---|---|---|---|
| ROX index at NIV initiation | 70 | 59.6 | 5.65 | 0.696 (0.599-0.792) | <0.001 |
| ROX index at 12h of NIV | 74.5 | 59.6 | 5.68 | 0.708 (0.612-0.804) | <0.001 |
| ROX index at 24h of NIV | 73.5 | 65.4 | 5.86 | 0.718 (0.621-0.815) | <0.001 |
| ROX index at 36h of NIV | 74.5 | 71.2 | 5.86 | 0.745 (0.649-0.841) | <0.001 |
| ROX index at 48h of NIV | 85.3 | 80 | 5.71 | 0.812 (0.727-0.897) | <0.001 |
| ROX index at 60h of NIV | 85.3 | 79.2 | 5.74 | 0.800 (0.713-0.888) | <0.001 |
| ROX index at 72h of NIV | 88.1 | 80 | 5.74 | 0.841 (0.763-0.919) | <0.001 |
Note: AUC - area under the ROC curve; CI - confidence interval; NIV - non-invasive ventilation; ROX index - ratio of pulse oximetry/fraction of inspired oxygen to respiratory rate.
Discussion
The respiratory support in COVID-19 is dependent on the severity of the disease and can be provided by using nasal cannulas, oxygen masks, high-flow nasal cannulas (HFNC), non-invasive positive pressure ventilation (NIPPV) (CPAP, Bi-PAP, PSV), and MV. During the pandemic, many clinical researches regarding non-invasive support applicability and influence on outcome in COVID-19 patients were performed, and different results were registered. Most of them encourage the use of non-invasive ventilation support [9]. The successful early NIV was evaluated in the Recovery-RS Clinical Trial, which demonstrated a decrease in mortality when using CPAP therapy as an initial respiratory support strategy compared to conventional oxygen therapy [10].
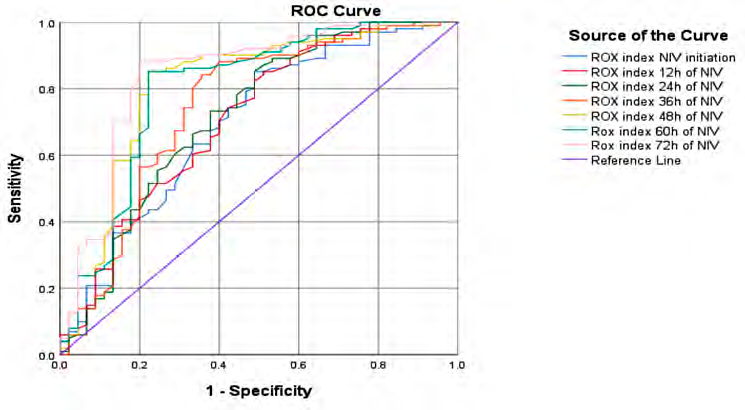
Fig. 2 ROC curve analysis for the ROX index values during non-invasive ventilation.
Overall, the rate of NIV use in COVID-19 patients is 25.5%-46%, with a failure rate between 30% and 88%. The registered mortality rate in non-success cases is around 59.8% [11-13]. Among the factors that may influence the negative result of non-invasive ventilation are: age > 60 years, comorbidities, low PaO2 / FiO2 ratio, low basal PaO2, CRP value, platelet count, respiratory rate, minute volume, ventilator ratio, D-Dimers level [12]. In this study, the risk for NIV failure was associated with age > 60 years, presence of hypertension, association of delirium and need for sedation during NIV. One of the factors that must be considered in COVID-19 patients at hospital admission is ISARIC score, which was developed, validated, and applied in 9 regions of the United Kingdom. This score evaluates 11 parameters at hospital admission or at first contact with patient. These are the number of comorbidities, age, and sex, presence of pulmonary infiltrates, urea level, respiratory rate, CRP, lymphocyte number, and oxygen saturation [14]. In the presented research, the patients with higher ISARIC mortality and deterioration score were more exposed to NIV failure.
The risk factors and laboratory parameters that influence the result of non-invasive ventilation that have been identified in our study are neutrophil/lymphocyte ratio during non-invasive support, lymphocyte count at 72-96h of NIV, leukocyte count at 24-48h and 72-96h of NIV, urea level during hospitalization, CRP at 24-48h of 72-96h of NIV, D-dimers level at 72-96h of NIV. All these factors, with varying degrees and according to different sources, reflect the clinical evolution, outcome, and prognosis of COVID-19. According to the previous publications, lymphopenia indicates a severe course of COVID-19 disease, due to increased viremia and consumption of immune cells, where the net number of lymphocytes is inversely proportional to the severity of the disease [15].
The previous publication highlighted that the neutrophil-lymphocyte ratio at a value higher than 9.8, indicates the high incidence of ARDS and the need for non-invasive or invasive ventilatory support [16]. The data recorded in this study suggest that neutrophil/lymphocyte ratio (at NIV initiation, at 24-48h of NIV and at 72-96h NIV) and values > 9.8 of N/L ratio represent the risk for NIV failure.
C-reactive protein is the inflammatory marker of the acute phase, and is produced by hepatocytes following stimulation by interleukin-6 and is used as an indicator of the severity of both inflammatory and infectious processes [17]. In the case of patients with COVID-19, it not only correlates with the degree and extent of pulmonary damage in the initial stage and the early pulmonary phase [18] but also suggests the possibility of poor prognosis and a four-fold higher rate of negative outcome and respiratory worsening at values more than 10 mg/l [19]. Our data identified increased values of CRP in the NIV failure group at 24-48h and 72-96h and the presence of values > 36mg/l were associated with NIV failure.
The presented study showed two-fold values of neutrophil/lymphocyte ratio in dynamics and CRP at 24-48h and 72-96h were identified in the group of patients with NIV failure. This suggests, that increased values of the neutrophil/lymphocyte ratio, CRP and the low number of lymphocytes during NIV indicate the lack of regression of the hyper-inflammatory process, whose evolution is closely correlated with the success of NIV.
The identified high levels of leukocytes in the group of patients with NIV failure suggest an association of bacterial superinfection in this group of patients, which has a rate of 24% in COVID-19 patients, and 41% in case of patients in ICU. The most commonly cultivated germs are Acinetobacter spp. (22.0%), Pseudomonas (10.8%), and Escherichia coli (6.9%) [20]. The presence of bacterial superinfection in patients with COVID-19 disease is an unfavorable prognostic factor, associated with an increased risk of mortality.
Because of the renal tropism of SARS-COV-2 virus [21], acute kidney injury is recorded at approx. 20% COVID-19 patients, with a mortality rate of approx. 55% in case of its association [22].
Urea values higher than 6.5 mmol/l indicate bad evolution and prognosis and a greater risk of developing the severe and critical form of the illness [23]. The urea values that were related to the risk of NIV failure in this investigation were > 7.5 mmol/l at admission, NIV initiation, and 24-48h of NIV.
Now, there is no consensus on the decision about conversion to mechanical ventilation, this action depends on national or local protocols and tactics, with a lack of global consensus on early or late intubation. These controversies are based on the lack of correlation between the clinical presentation, imaging or the PaO2 / FiO2 ratio used to stratify the severity degree of classic ARDS. For this reason, it has been proposed to manage these patients based on their clinical phenotype [24, 25]. At the same time, the difference in mortality depending on the timing of the intubation has not been proven yet. This justifies the continued application of the wait-and-see approach in some of the clinics [26].
In COVID-19 patients, remain uncertain the criteria and indications for the initiation of mechanical ventilation are. In more of the cases, they are progression of respiratory distress with signs of tissue hypoxia, PaO2 value <50 mmhg, severe acidosis pH <7.25, work of breathing and delirium [27]. Nevertheless, on the other hand, the wait-and-see approach has led to appearance of multiple discussions around the phenomenon of P-SILI (patient self-inflicted long injury) which lung injury is induced by the patient's own respiratory effort [28].
One of the predictors of non-invasive support techniques failure is ROX Index, which is used for the prediction of the HFNC failure in patients with COVID-19 ARF [29]. Dynamically evaluated every 12 hours, ROX index indicates a high risk of failure of non-invasive ventilation, the need for intubation and mechanical ventilation when its value decrease below 5.99 (more specifically for COVID-19 patients) or 4.88 (in non-covid-19 patients) [30]. The ROX index values that were recorded in this survey were predictive from NIV initiation and during 72h of NIV and may warn about respiratory worsening and the need to discuss the conversion to mechanical ventilation when evaluated in dynamics every 12 hours.
In presented research the reported mortality rate in case of NIV failure was 96%. This may be related to the fact that the study included patients from the first period of the pandemic, this being associated with the lack of experience in medical management and respiratory support.
Conclusions
The abnormal values during continuous measurement of laboratory parameters such as neutrophil/lymphocyte ratio, urea level, lymphocyte count, increase in WBC count and maintaining of high values of CPR and D-dimer, as well as association of delirium and need for sedation during NIV, can alert and inform clinicians about the risks of NIV failure in COVID-19 patients with acute respiratory failure. ROX index follow-up every 12h from NIV initiation and through 72h of NIV may predict respiratory worsening in non-invasive ventilated patient. Continuous measurement of these parameters may help the clinicians to decide the optimal timing of conversion to invasive ventilation.
Competing interests
None declared.
Authors' contribution
The authors contributed equally to the research of the scientific literature, the selection of the bibliography, the reading, and analysis of biographical references, the writing of the manuscript and its peer review. All authors have read and approved the final version of the article.
Acknowledgements and funding
The authors would like to acknowledge the support of all medical staff of COVID-19 ICU of the Institute of Emergency Medicine. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Patient consent
Obtained.
Ethics approval
The study protocol was approved by the Research Ethics Committee of Nicolae Testemițanu State University of Medicine and Pharmacy (minutes No.4 from 07.07.2021).
Authors’ ORCID IDs
Ivan Cîvîrjic – https://orcid.org/0000-0002-1360-5485
Alina Nerpii – https://orcid.org/0009-0005-6237-8265
Natalia Stefantov – https://orcid.org/0009-0006-9390-6099
Ina Voleac – https://orcid.org/0009-0001-1137-2243
Natalia Cernei – https://orcid.org/0000-0002-2031-5881
Olga Gherasim – https://orcid.org/0000-0002-8184-4912
Serghei Șandru – https://orcid.org/0000-0002-2973-9154
References
- Worldometer [Internet] COVID-19. Coronavirus Statistics. ©2020- [cited 2023 Mar 12]. Available from: https://www.worldometers.info/coronavirus/
- Li J, Huang DQ, Zou B, Yang H, Hui WZ, Rui F, et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2021 Mar 1;93(3):1449-58. doi: 10.1002/jmv.26424.
- Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020 Mar 12;14(2):185-92. doi: 10.1007/s11684-020-0754-0.
- Behrens EM, Koretzky GA. Review: Cytokine storm syndrome: looking toward the precision medicine era. Arthritis Rheumatol. 2017 Jun 1;69(6):1135-43. doi: 10.1002/ art.40071.
- Shi C, Wang L, Ye J, Gu Z, Wang S, Xia J, et al. Predictors of mortality in patients with coronavirus disease 2019: a systematic review and meta-analysis. doi: 10.1186/s12879-021-06369-0.
- Tootsiz OS, Fischer B, Fischer H, Zeintlgr H. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care. 2020 Aug 21;24(1):516. doi: 10.1186/s13054-020-03240-7.
- Morales-GanúE.L.RoficoValverde-LeóM.González-Gaspar J. Rodríguez-Muñoz PM, Hidalgo-Lopezosa P, Rodríguez-Borrego MA, et al. Ventilatory therapies in emergency care admission for patients with COVID-19: A systematic review. Rev Med Pharmacol Sci. 2021;25(6):2730-43. doi:10.26355/eurrev_202103_25436.
- Grover S, Avasthi A. Clinical practice guidelines for management of delirium in elderly. Indian J Psychiatry. 2018 Feb 1;60(Suppl 3):S329-S340. doi: 10.4103/0019-5545.224473.
- Ogawa K, Asano K, IkedaJ, Fujii T. Non-invasive oxygenation strategies for respiratory failure with COVID-19: a concise narrative review of literature in pre and mid-COVID-19 era. Asian Crit Care Pain Med. 2021 Aug 1;40(4):100897. doi: 10.1016/j.accpm.2021.100897.
- Perkins GD, Ji C, Connolly BA, Couper K, Lall R, Baillie JK, et al. Effect of noninvasive respiratory strategies on intubation or mortality among patients with acute hypoxemic respiratory failure and COVID-19: the RECOVERY-RS randomized clinical trial. JAMA. 2022 Feb 8;327(6):546-58. doi: 10.1001/jama.2022.0028.
- Radovanovic D, Coppola S, Franceschi E, Gervasoni F, Duscio E, Chiumello DA, et al. Mortality and clinical outcomes in patients with COVID-19 pneumonia treated with non-invasive respiratory support: a rapid review. J Crit Care. 2021 Oct 1;65:1-8. doi: 10.1016/j.jcrc.2021.05.007.
- Groff P, Ferrari R. Non-invasive respiratory support in the treatment of acute hypoxemic respiratory failure secondary to CoVid-19 related pneumonia. Eur J Intern Med. 2021 Apr 1;86:17-21. doi: 10.1016/j.ejim.2021.02.015.
- Serafini RB, Póvoa P, Souza-Dantas V, Kall AC, Salluh JIF. Clinical outcomes and outcomes of critically ill patients with COVID-19 infection: a systematic review. Clin Microbiol Infect. 2021 Jan 1;27(1):47-54. doi: 10.1016/j.cmi.2020.10.017.
- Gupta RK, Harrison EM, Ho A, Docherty AB, Knight SR, van Smeden M, et al. Development and validation of the ISARIC 4C deterioration model for adults hospitalized with COVID-19: a prospective cohort study. Lancet Respir Med. 2021 Apr 1;9(4):349-59. doi: 10.1016/S2213-2600(20)30559-2.
- Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020 Mar 27;5(1):1-3. doi: 10.1038/s41392-020-0148-4.
- Ma A, Cheng J, Yang J, Dong M, Liao X, Kang Y. Neutrophil-to-lymphocyte ratio as a predictive biomarker for moderate-severe ARDS in severe COVID-19 patients. Crit Care. 2020 Jun 5;24(1):33. doi: 10.1186/s13054-020-03007-0.
- Chalmers S, Khawaja A, Wieruszewski PM, Gajic O, Odeyemi Y. Diagnosis and treatment of acute pulmonary inflammation in critically ill patients: the role of inflammatory biomarkers. World J Crit Care Med. 2019 Sep 11;8(5):59-71. doi: 10.5492/wjccm.v8.i5.59.
- Tan C, Huang Y, Shi F, Tan K, Ma Q, Chen Y, et al. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J Med Virol. 2020 Jul 1;92(7):856-62. doi: 10.1002/jmv.25871.
- Malik P, Patel U, Mehta D, Patel N, Kelkar R, Akrmah M, et al. Biomarkers and outcomes of COVID-19 hospitalizations: systematic review and meta-analysis. BMJ Evid Based Med. 2021.