Introduction
Intrahepatic cholestasis of pregnancy (ICP), also known as cholestasis gravidarum, is a liver disease with a global incidence of 0.5-1% [1]. The onset of cholestasis gravidarum is characterized by the appearance of cutaneous pruritus, typically on the palms and legs that cannot be explained by other factors [2].
The etiology and pathogenesis of cholestasis gravidarum are multifactorial, depending on environmental factors, hormonal changes, and genetic variations [3]. It should be noted that the diagnosis of intrahepatic cholestasis of pregnancy is one of exclusion and should be differentiated from other pregnancy-related liver pathologies, which may have similar laboratory results, such as preeclampsia, acute fatty liver of pregnancy, and HELLP syndrome [4].
The standard diagnostic criterion in ICP remains the assessment of serum bile acid (BA) levels (BA ≥10 µmol/L) and liver function test (LFT) results [5]. The assessed serum BA levels are considered the definitive biochemical markers in the diagnosis of ICP, and they are also used to monitor the condition of patients [2]. Based on BA values, cholestasis gravidarum can be classified as being mild (BA 10-39 μmol/L) and severe (BA ≥40 μmol/L) [6]. In ICP, alanine aminotransferase values increase about 2-10 times, being a more sensitive marker of ICP compared to aspartate aminotransferase, the values of which do not significantly increase in women with this condition [7]. There is data in the literature suggesting increased total bilirubin levels in about 10% of cases complicated by ICP, although its values rarely exceed 85.5 μmol/L [8, 9]. Serum ɣ-glutamyltransferase levels decrease during pregnancy, while alkaline phosphatase activity increases due to placental isoenzyme production and increased bone isoenzyme activity. Nevertheless, simultaneous increases in ɣ-glutamyltransferase and alkaline phosphatase indicate liver pathology [10].
There is a discrepancy in the literature regarding the onset of ICP and whether it is signified by an increase in BA and LFT values or the onset of clinical symptoms. In some studies, the majority of patients were primarily diagnosed with increased BA levels before presenting clinical symptoms or other LFT changes [7]. Other studies have described cases of cholestasis gravidarum in which clinical symptoms have been observed at the onset of the pathology, with changes in BA and LFT values only occurring after 4-5 weeks [11]. Following delivery, clinical symptoms of cholestasis gravidarum resolve in most cases within 48 hours, with LFT values returning to normal over 2-8 weeks postpartum [3, 12].
Recent studies have demonstrated the prognostic role of hematological inflammatory markers in both cardiovascular diseases and malignancies [13, 14]. At the same time, there are few studies that have focused on studying hematological inflammatory markers in intrahepatic cholestasis of pregnancy [3]. Considering that bile acids are cytotoxic substances; their high values induce the release of proinflammatory mediators [15].
Some authors suggest that in ICP, women experience specific changes in hematological inflammatory markers, including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), mean platelet volume (MPV), red cell distribution width – standard deviation (RDW-SD) and red cell distribution width – coefficient of variation (RDW-CV), although the results are inconclusive. Recently, NLR was found to be a prospective diagnostic marker in assessing the severity of ICP [16]. However, these data have not been refuted by other researchers who did not detect a difference between NLR values in women whose pregnancy was complicated by ICP compared to a control group [3]. Abide.Y. et al. discovered that MPV values increase significantly in cholestasis gravidarum, correlating with the pathology’s severity [3]. Hence, studying hematological inflammatory markers may be a promising diagnostic method in assessing the severity of intrahepatic cholestasis of pregnancy.
Material and methods
A prospective study was conducted during 2020-2022 in three institutions, including the Department of Obstetrics and Gynecology of the Nicolae Testemitanu State University of Medicine and Pharmacy, the Institute of Mother and Child, and the Clinical Hospital „Gheorghe Paladi” in Chisinau, Republic of Moldova. The study protocol was approved by the Research Ethics Committee of the Nicolae Testemitanu State University of Medicine and Pharmacy on April 17, 2020.
The representative research sample was calculated using EpiInfo 7.2.2.6 in the StatCalc Sample Size and Power section based on the following parameters:
Confidence interval for 95.0% significance of results;
Statistical power - 80.0%;
The difference in the course and outcome of pregnancy in pregnant women with intrahepatic cholestasis of pregnancy compared to pregnant women without ICP constitutes on average up to 20.0% [17];
Ratio between the investigated groups = 1:1;
Result: for the 95.0% CI the calculated value is 44 with 10.0% non-response rate, n=48;
Therefore, there must be no less than 48 pregnant women with ICP in the research group;
Two groups were created for the prospective research:
Group A - 71 pregnant women whose pregnancy was complicated by intrahepatic cholestasis of pregnancy (main group);
Group B - 71 pregnant women whose pregnancies were not complicated by intrahepatic cholestasis of pregnancy (control group).
The research was carried out by assessing the BA levels and hematological inflammatory markers in the blood of participants, as well as by studying medical records (obstetric medical observation form no. 96/e). The diagnosis of ICP was established on the basis of anamnestic, clinical, and biochemical data. Biochemical analysis of blood was performed using an Abbott Architect c8000 analyzer. The NLR and PLR values were calculated using standard formulas. NLR was calculated by dividing the absolute number of neutrophils by the absolute number of lymphocytes [18, 19]. PLR was calculated by dividing the absolute number of platelets by the absolute number of lymphocytes [19]. The rest of the hematological markers were calculated automatically using an automated hematological analyzer - SYSMEX XN-1000.
The statistical data were processed using IBM Statistics SPSS 21 and the QuickCalcs section of GraphPad. Arithmetic mean and standard deviation (M (SD)) values were calculated to describe numerical indicators. To assess distributions of characteristics that differed from normal the median (Me) and the interquartile range (Q1; Q3) were calculated. For comparison of categorical variables by groups, the χ² test without Yattes' continuity correction was used; a p-value<0.05 was considered statistically significant.
Results
The mean age was 29.5 (0.7) years (Me 30 (25; 34)) for women in group A and 27.3 (0.6) years (Me 27 (23; 31)) in group B. The age of the pregnant women included in the study ranged from 18-43 years, while 16/71 (22.5%) women in group A vs. 8/71 (11.3%) women in group B were aged over 35 years. All of the women in the study were included in their family doctor’s register as having a current pregnancy, with the majority having seen their family doctor early in pregnancy. In group A, 41/71 (57.7%) women were multiparous, compared to 34/71 (47.8%) in group B. The study found that 15/142 (10.5%) pregnant women had developed cholestasis gravidarum in previous pregnancies, 14/71 (19.7%) of whom were in group A and 1/71 (1.4%) were in group B (χ2 12.597, p=0.0004).
In pregnant women in group A, multiple pregnancies occurred in 8/71 (11.3%) cases, of which 6/8 were twin pregnancies and 2/8 were pregnancies with triplets. In the control group, multiple pregnancy occurred in 4/71 (5.6%) pregnant women (χ2 1.456, p=0.2275), 2/4 cases being with twins and 2/4 with triplets. Of note is that 5/71 (7.0%) pregnancies in group A were achieved by in vitro fertilization (IVF) compared to 3/71 (4.2%) cases of IVF in group B (χ2 0.530, p=0.4667).
Cutaneous pruritus of varying location and intensity was experienced by all women in group A (Table 1), being the main clinical symptom of cholestasis gravidarum. At the same time, 32/71 (45.1%) women from group A reported skin pruritus simultaneously in several parts of the body. While some women from group B (7/71 (9.8%) experienced occasional skin pruritus, which in most cases was localized in the abdominal region, participants explained symptoms to have resulted from an increase in pregnancy term, topical application of different cosmetic substances, and discomfort caused by underwear.
Table 1. Location of cutaneous pruritis in women included in the study. | |||
| Location of cutaneous pruritis | Group A, n=71 (abs., %) | Group B, n=71 (abs., %) |
1 | Pruritus with localization on palms | 25 (35.2%) | 1 (1.4%) |
2 | Pruritus with localization on legs | 23 (32.4%) | 0 |
3 | Pruritus with localization on abdomen | 18 (25.4%) | 5 (7.0%) |
4 | Generalized cutaneous pruritus | 37 (52.1%) | 1 (1.4%) |
Note: the total percentage exceeds 100% as some women experienced more than one symptom at the same time. | |||
In order to assess the severity of intrahepatic cholestasis of pregnancy, serum BA levels were assessed in women from both study groups. In group A, the BA levels ranged from 10 – 211.3 µmol/L, the mean value was 34.7 (4.3) µmol/L (Me 18.9 (11.1; 44.0)). Hence, mild ICP was found in 50/71 (70.4%) cases and severe ICP in 21/71 (29.6%) cases. The mean value of BA in group B was 3.3±0.1 µmol/L.
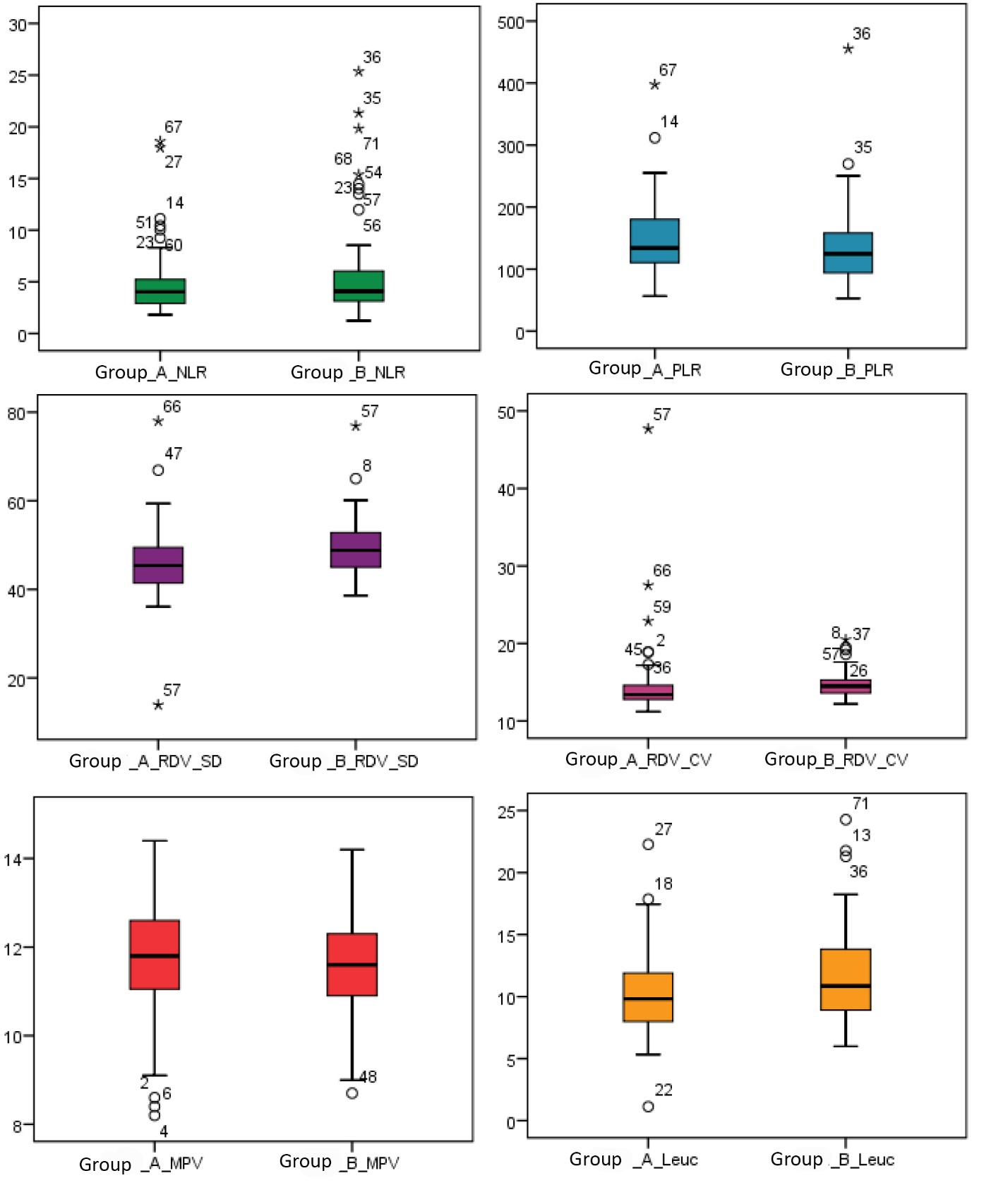
It was of interest to study the levels of hematological inflammatory markers in the women included in the study (Figure 1). Thus, mean NLR values were 4.7 (0.3) (Me 4.0 (2.9; 5.2)) in group A and 5.6 (0.5) (Me 4.0 (3.1; 6.1)) in group B. Mean PLR values were 146.0 (6.8) (Me 134.0 (110.2; 180.2)) in group A compared to 135.2 (7.3) (Me 124.7 (93.7; 158.2)) in group B. Mean values of MPV in group A were 11.6 (0.1) fl (Me 11.8 (11.0; 12.6)); in group B mean values of this marker were 13.3 (1.7) fl (Me 11.6 (10.9; 12.3)). The mean values of erythrocyte distribution indices were: RDW-SD 45.9 (0.9) fl (Me 45.4 (41.4; 49.5)) and RDW-CV 14.6 (0.5)% (Me 13.4 (12.8; 14.6)) in group A compared to RDW-SD 49.0 (0.7) fl (Me 48.8 (44.9; 53.1)) and RDW-CV 14.7 (0.1)% (Me 14.5 (13.6; 15.3)) in group B. Likewise, mean leukocyte (leuc.) values were 10.2 (3.3) x103/μL (Me 9.8 (7.9; 11.9)) in group A compared to group B, where they were 11.8 (3.8) x103/μL (Me 10.8 (8.7; 13.8)).
In order to assess the role of hematological inflammatory markers in the severity of intrahepatic cholestasis of pregnancy, women in group A were divided into two subgroups based on serum BA levels. The levels of hematological inflammatory markers in each subgroup were then analyzed (Table 2). A significant increase in PLR values was detected in severe ICP cases (159.3 (11.6)) compared to mild cases of the condition (140.4 (8.4)), correlating with the severity of the pathology.
Discussions
The findings are consistent with the literature, which shows that cholestasis gravidarum occurs more frequently in pregnant women with twin pregnancies than in singleton pregnancies (2.1% vs. 0.3%), also linked to an increased rate of ICP in IVF pregnancies (2.7%) [20, 21]. Some authors suggest that this pathology more often affects patients older than 35 years [22]. In the current study, 9.3% of women in the control group experienced cutaneous pruritus, whereas in the general population, according to literature data, up to 23% of pregnant women with physiological pregnancy experience cutaneous pruritus [11].
|
Fig. 1 The level of hematological inflammatory markers in women included in the study. Note: NLR – neutrophil-to-lymphocyte ratio; PLR – platelet-to-lymphocyte ratio; MPV – mean platelet volume; RDV-SD – red cell distribution width – standard deviation; RDV-CV – red cell distribution width – coefficient of variation; leuc. – leucocyte. |
Assessment of BA levels is extremely important for patients with ICP, not only because of the maternal impact of the condition, but also in terms of perinatal outcomes related to cholestasis gravidarum. Glantz A. et al. reported an increased rate of adverse perinatal outcomes in pregnant women with serum BA levels >40 μmol/L [23]. The same study reported a 1-2% increase in the risk of spontaneous preterm birth, fetal asphyxia, or the presence of meconium in the amniotic fluid, with an increase for each additional µmol/L of BA above 40 μmol/L [9]. A recent study suggests that in cases of ICP with BA values >40 μmol/L, women give birth on average two weeks earlier than pregnant women in the control group [24]. In a recent meta-analysis of published studies, perinatal outcomes in pregnant women with ICP were evaluated and it was found that extremely high serum BA levels (>100 μmol/L) significantly increase the risk of intrauterine fetal death [25].
Table 2. The mean values of hematological inflammatory markers in women included in the study. | ||||
| Hematological inflammatory markers | Group A n=71 | Group B n=71 M (SD) | |
Subgroup I Mild ICP n=50 M (SD) | Subgroup II Severe ICP n=21 M (SD) | |||
1 | NLR | 4.8 (0.4) Me 3.9 (2.9; 5,4) | 4.3 (0.5) Me 4.0 (2,3;5,0) | 5.6 (0.5) Me 4.0 (3.1; 6.1) |
2 | PLR | 140.4 (8,4) Me 126.4 (103.5; 170.7) | 159.3 (11.6) Me 158.4 (119.,8; 184.3) | 135.2 (7.3) Me 124.7 (93.7; 158.2) |
3 | MPV, fl | 11.8 (0.1) Me 12.0 (11.1; 12.6) | 11.3 (0.3) Me 11.5 (9.9; 12.7) | 13.3 (1.7) Me 11.6 (10.9; 12.3) |
4 | RDW-SD, fl | 46.2 (1.0) Me 44.9 (41.3; 49.4) | 45.1 (1.9) Me 47.0 (41.7; 50.0) | 49.0 (0.7) Me 48.8 (44.9; 53.1) |
5 | RDW-CV, % | 14.2 (0.3) Me 13.4 (12.7; 14.6) | 15.5 (1.6) Me 13.3 (12.8; 14.7) | 14.7 (0.1) Me 14.5 (13.6; 15.3) |
Note: NLR – neutrophil-to-lymphocyte ratio; PLR – platelet-to-lymphocyte ratio; MPV – mean platelet volume; RDV-SD – red cell distribution width – standard deviation; RDV-CV – red cell distribution width – coefficient of variation. | ||||
Despite the fact that the assessment of the NLR level is used to predict the severity of different pathologies, there is no conclusive opinion in the literature regarding NLR reference values, as they are dependent on several factors, such as age, gender, etc. However, there are studies in which authors have compared NLR and PLR levels in physiological pregnancies and those complicated by pre-eclampsia, reporting an increase in these parameters in cases of pre-eclampsia [26, 27]. However, the data presented is inconclusive, thus requiring further research.
The results of the study conducted by Abide Ç. Y. et al. showed that BA levels correlated positively and significantly with PLR (r=0.343, p=0.003) and women whose pregnancies were complicated by ICP showed significantly increased PLR values compared to the control group [3]. MPV, which is the most widely used measure of platelet size, is also an index of platelet activation. Platelets release thrombin, which plays a role in inflammation and angiogenesis. At the same time, a large platelet volume leads to increased coagulability and fibrinolysis [28, 29]. There are a limited number of studies that have focused on the correlation between MPV and ICP severity and on the correlation between MPV and perinatal outcomes, although an increase in MPV may be observed in women with ICP [3, 30]. Moreover, one study found that MPV correlates with ICP severity and could be a valuable marker for assessing the severity of the condition [3].
Conclusions
The study of hematological inflammatory markers showed a significant increase in platelet-to-lymphocyte ratio in women whose pregnancy was complicated by ICP. Moreover, the study data show an increase in PLR values in severe intrahepatic cholestasis of pregnancy. The values of NLR and MPV were similar in the group of women with ICP and in the control group. RDW-SD and RDW-CV values were lower in the group with cholestasis gravidarum compared to the control group. Thus, given these results, we can conclude that PLR can be a promising diagnostic marker in assessing the severity of ICP.
Competing interests
None declared
Authors’ contributions
The authors have equally contributed to the manuscript drafting, design and editing. The final version of the manuscript was approved by all authors.
Authors’ ORCID IDs
Maria Cemortan - https://orcid.org/0000-0003-3137-7524
Olga Cernețchi - https://orcid.org/0000-0002-9229-8080
References
Ozkan S., Ceylan Y., Ozkan O.V., Yildirim S. Review of a challenging clinical issue: Intrahepatic cholestasis of pregnancy. In: World J. Gastroenterol. 2015; 21(23): 7134-7141. Disponibil pe: URL: http://www.wjgnet.com/1007-9327/full/v21/i23/7134.htm DOI: http://dx.doi.org/10.3748/wjg.v21.i23.7134
Williamson C., Geenes V. Intrahepatic cholestasis of pregnancy. In: Obstet. Gynecol. 2014; 124: 120-133 [PMID: 24901263 DOI:10.1097/AOG.0000000000000346]
Abide Ç.Y., et al. Can we predict severity of intrahepatic cholestasis of pregnancy using inflammatory markers? In: Turkish Journal of Obstetrics and Gynecology. 2017; 14(3): 160.
Geenes V., Williamson C. Liver disease in pregnancy. In: Best Pract. Res. Clin. Obstet. Gynecol. 2015; 29: 612-624.
Kenyon A.P., Piercy C.N., Girling J., et al. Pruritus may precede abnormal liver function tests in pregnant women with obstetric cholestasis: a longitudinal analysis. In: BJOG. 2001; 108: 1190-1192.
Smith D.D., Rood K.M. Intrahepatic Cholestasis of Pregnancy. In: Clinical Obstetrics and Gynecology. 2020; 63(1): 134-151.
- Gabzdyl E.M., Schlaeger J.M. Intrahepatic cholestasis of pregnancy: a critical clinical review. In: J. Perinat. Neonatal Nurs. 2015; 29: 41-50. [PMID: 25633399 https://doi.org/10.1097/JPN.0000000000000077 ]
Wood Amber M., et al. Intrahepatic cholestasis of pregnancy: a review of diagnosis and management. In: Obstetrical & Gynecological Survey. 2018; 73(2): 103-109. Disponibil pe: https://doi.org/10.1097/ogx.0000000000000524
Geenes V., Williamson C., Chappell L.C. Intrahepatic cholestasis of pregnancy. In: The Obstetrician & Gynaecologist. 2016; 18(4): 273-281. Disponibil pe: https://doi.org/10.1111/tog.12308
Ammon F.J., Kohlhaas A., Elshaarawy O., et al. Liver stiffness reversibly increases during pregnancy and independently predicts preeclampsia. In: World Journal of Gastroenterology. 2018; 24(38): 4393. Disponibil pe: https://doi.org/10.3748/wjg.v24.i38.4393
Morton A., Laurie J. The biochemical diagnosis of intrahepatic cholestasis of pregnancy. In: Obstetric Medicine. 2019; 12(2): 76-78.
Brouwers L., Koster M.P., Page-Christiaens G.C., et al. Intrahepatic cholestasis of pregnancy: maternal and fetal outcomes associated with elevated bile acid levels. In: Am. J. Obstet. Gynecol. 2015; 212: 100.e1-e7.
Nunez J., Nunez E., Bodi V., et al. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. In: Am. J. Cardiol. 2008; 101: 747-752.
Zhang W.W., Liu K.J., Hu G.L., Liang W.J. Preoperative platelet/lymphocyte ratio is a superior prognostic factor compared to other systemic inflammatory response markers in ovarian cancer patients. In: Tumour Biol. 2015; 36: 8831-8837.
Cai S., Li M., Boyer J.L. The Role of Bile Acid‐Mediated Inflammation in Cholestatic Liver Injury. In: The Liver. 2020; 728–736. Disponibil pe: doi:10.1002/9781119436812.ch56
Kirbas A., Biberoglu E., Daglar K., et al. Neutrophil-to-lymphocyte ratio as a diagnostic marker of intrahepatic cholestasis of pregnancy. In: Eur. J. Obstet. Gynecol. Reprod. Biol. 2014; 180: 12-15.
- Еремина Е.Ю., Машарова А.А. Внутрипеченочный холестаз у беременных. B: Экспериментальная и клиническая гастроэнтерология. 2011; 6.
- Forget P., Khalifa C., Defour JP., et al. What is the normal value of the neutrophil-to-lymphocyte ratio? In: BMC Res. Notes. 2017; 10: 12. Disponibil pe: https://doi.org/10.1186/s13104-016-2335-5
Dharmapuri S., et al. Predictive value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in advanced hepatocellular carcinoma patients treated with anti-PD‐1 therapy. In: Cancer Medicine. 2020; 9(14): 4962-4970.
Вороник Ю.Н., Мацюк Я.Р. Холестаз беременных: этиопатогенез, лечение и прогноз (обзор). B: Вестник Смоленской государственной медицинской академии. 2018; 3.
Radu-Ionita F., Pyrsopoulos N.T., Jinga M., et al. (Eds.). Liver Diseases. 2020. Disponibil pe: doi:10.1007/978-3-030-24432-3
Manzotti C., et al. Total serum bile acids or serum bile acid profile, or both, for the diagnosis of intrahepatic cholestasis of pregnancy. In: Cochrane Database of Systematic Reviews. 2019; 7.
Glantz Anna, Hanns‐Ulrich Marschall, Lars‐Åke Mattsson. Intrahepatic cholestasis of pregnancy: relationships between bile acid levels and fetal complication rates. In: Hepatology. 2004; 40.2: 467-474.
Guszczynska-Losy M., et al. Evaluation of predictive value of biochemical markers for adverse obstetrics outcomes in pregnancies complicated by cholestasis. In: Ginekologia Polska. 2020; 91(5): 269-276.
Ovadia C., Seed P.T., Sklavounos A., et al. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: results of aggregate and individual patient data meta-analyses. In: Lancet. 2019; 393: 899–909.
Kirbas A., Ersoy A.O., Daglar K., et al.. Prediction of preeclampsia by first trimester combined test and simple complete blood count parameters. In: Journal of Clinical and Diagnostic Research. 2015; 9: QC20–QC23.
Gezer C., Ekin A., Ertas I.E., et al. High first-trimester neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios are indicators for early diagnosis of preeclampsia. In: Ginekologia Polska. 2016; 87: 431–435.
Kisucka J., Butterfield C.E., Duda D.G., et al. Platelets and platelet adhesion support angiogenesis while preventing excessive hemorrhage. In: Proc. Natl. Acad. Sci. USA. 2006; 103: 855-860.
Ross R. Atherosclerosis: an inflammatory disease. In: N. Engl. J. Med. 1999; 340: 115-126.
Oztas E., Erkenekli K., Ozler S., et al. Can routine laboratory parameters predict adverse pregnancy outcomes in intrahepatic cholestasis of pregnancy? In: J. Perinat. Med. 2015; 43: 667-674.