Introduction
One of the difficult problems of internal medicine are intestinal infectious diseases (IID). The IID clinical picture is extremely diverse, therefore they should be considered as systemic diseases. The lesions, unfortunately, are not limited only to the gastrointestinal tract; some of the patients develop extraintestinal manifestations [1-3].
Extraintestinal manifestations are separated into two distinct pathogenetic groups: immune-mediated diseases due to a common pathogenetic link and non-immune-mediated diseases, its clinical picture due to metabolic processes that are secondary to intestinal infectious disease. The first group includes arthropathy, skin lesions (erythema nodosum, gangrenous pyoderma), eye damage (uveitis, iridocyclitis), primary sclerosing cholangitis; in the second group - cholelithiasis, urolithiasis, osteoporosis [4-6].
According to clinical studies, up to half of patients with IID suffer from at least one extraintestinal manifestation, and in some patients, it occurs before the onset of intestinal symptoms, which causes additional difficulties in the early diagnosis of the disease. The presence of one or more extraintestinal immune-mediated manifestations changes the prognosis, increases the severity the disease, and modifies the management [7-9].
Arthropathy, lesions of the skin, eyes, and mucous membranes are among the most common extraintestinal manifestations. Various cellular and humoral mechanisms have been identified that account for intestinal and joint inflammation. Damage to peripheral joints and spine is the most common manifestation of associated spondyloarthritis. Axial spondyloarthritis (axSpA) belongs to the group of spondyloarthritis, which includes ankylosing spondylitis (AS), psoriatic arthritis, reactive arthritis, and arthritis associated with IID [10-12]. According to studies in patients with IID, the incidence of SpA ranges from 17% to 39%.
Establishing the diagnosis of IID-associated SpA at an early stage is a very difficult task: the clinical manifestations are minor, but the radiological manifestations are reliable and are usually detected on average 7.1 years after the onset of symptoms [13]. A group of Italian researchers reported that the diagnosis of SpA in patients with IID is after around 5.2 years from the onset of dorsal pain. Currently, the incidence of undiagnosed cases remains to be quite high [14, 15].
Despite numerous fundamental clinical studies on the frequency, pathogenesis, clinical features of SpA in IID and intestinal damage in AS, there are currently several open issues, one of which is the problem of early diagnosis of arthropathies, especially associated with IID. Therefore, it was decided to initiate a study with the following purpose – Determination of the peculiarities of early manifestations of axial arthropathies in intestinal infectious diseases in order to improve their early diagnosis.
The objectives of the study are:
identification of clinical variants of axial arthropathies in patients with infectious intestinal diseases and instrumental description of axial lesion;
conducting comparative analysis of clinical and laboratory parameters in patients with intestinal infectious disease, depending on the presence of axial arthropathies;
study of clinical features of gastrointestinal lesions in patients with ankylosing spondylitis;
determination of diagnostic value of ASAS criteria, 2009 for dorsal inflammatory pain in infectious bowel disease;
development of an algorithm for the early detection of axial spondyloarthritis in intestinal infectious diseases.
The scientific novelty of the study is – for the first time in patients with IID, the incidence of spinal inflammatory pain was determined (according to the ASAS criteria, 2009), which was 42.9%, which contributed to the early diagnosis of axSpA in 28.6% of patients. Furthermore, an attempt was made to determine the factors that are most commonly encountered in the presence of SpA in patients with IID. It has been determined that the presence of arthritis - odds ratio (OR) 10.77 [95% CI 2.26-44.2], p=0.005, arthralgia – OR 4.12 [95% CI 1.55-10.95], p = 0.005, inflammatory back pain - OR 8.07 [95% CI 2.8-23.23], p = 0.0001, uveitis - OR 19.2 [95% CI 2.18-169.13], p = 0.008 increases the risk of developing SpA in patients with IID. Patients with S±Sh have a higher risk of developing SpA compared to patients with Y±C – OR 2.92 [95% CI 1.14-7.48], p = 0.025.
We determined the diagnostic significance of the ASAS criteria of inflammatory pain in infectious bowel diseases. The sensitivity was 75.9%, the specificity – 68.3%, the positive predictive value – 0.51, the negative predictive value – 0.87; the probability ratio of the positive result – 2.3, the probability ratio of the negative result was 0.3. When using the DISQ questionnaire to identify intestinal symptoms in patients with AS, it was demonstrated for the first time that 92% of patients had remission of gastrointestinal symptoms.
Based on the obtained results, we proposed clinical predictors of the presence of axSpA in patients with IID. These include arthralgia, arthritis, inflammatory back pain, and uveitis. It is necessary to consider the early detection of SpA in patients with S±Sh. The diagnostic significance of 2009 ASAS classification criteria in patients with SpA and IID was determined, which allows them to be used in clinical practice. An algorithm is proposed for the early diagnosis of axSpA in patients with IID, which helps in identifying the opportune time for rheumatology referral. It should be taken into account that in patients with AS with a long course and severe activity, the manifestations on the part of the gastrointestinal tract are subclinical.
Material and methods
During 2015-2021, 141 patients were analyzed retrospectively. Of the 141 patients, 50 patients were with AS from the Republican Clinical Hospital „Timofei Moşneaga” and 91 patients were with infectious intestinal diseases from the gastro-enterology and hepatology departments of the Republican Clinical Hospital „Timofei Moşneaga”. Depending on the mediation of the inflammatory response, all patients with IID were divided into 2 groups: the first group included patients with Yersinia enterocolitica or Campylobacter jejuni (Y±C), the second group with Salmonella enteritidis or Shigella flexneri (S±Sh).
The criteria for inclusion in the study were:
age over 18 years;
the presence of an established diagnosis of inflammatory infectious disease;
a diagnosis of AS;
combination of IID with extraintestinal manifestations (including SpA);
signed informed consent for inclusion in the study.
Exclusion criteria:
pregnancy and lactation;
the patient's refusal to participate in the study.
The study included 91 patients with IID: 52 (57.1%) with Yersinia enterocolitica or Campylobacter jejuni (Y±C), and 39 (42.9%) with Salmonella enteritidis or Shigella flexneri (S+-Sh). There were 47 men (51.6%) and 44 women (48.4%); as well as 50 patients with AS: men – 33 (66%), women – 17 (34%). Diagnoses of Y±C, S±Sh have been established in accordance with modern recommendations for the corresponding nosological form. Back pain was considered inflammatory if it met the 2009 ASAS criteria for inflammatory low back pain (if at least 4 out of 5 criteria were met).
The diagnosis of AS was established according to the criteria of the 1984 New York amended criteria. The diagnosis of axSpA was established by a joint consultation between a gastroenterologist and rheumatologist based on typical SpA complaints (in particular, spinal inflammatory pain and changes detected by imaging methods – MRI and pelvic X-ray). Imaging data were considered positive if the patient had significant sacroiliitis (SI) (Ro+) (Van der Linden et al., 1984) or signs on MRI (MRI+). All patients diagnosed with IID-associated SpA met the ASAS classification criteria for axial SpA.
The examination of patients with IID and AS was based on the standard of examination of patients with Y±C, S±Sh, AS and recommendations for the corresponding nosological form. Additional methods of examination included the ASAS questionnaire for dorsal inflammatory disease (2009) in patients with IID, determination of fecal calprotectin and the DISQ questionnaire in patients with AS.
Statistical analysis
The nature of the distribution of the data obtained was evaluated using a graphical method using the Kolmogorov-Smirnov criterion. Descriptive statistical methods were used for the statistical processing of the research data. The description of signs that have a normal distribution is presented as M±δ, where M is the arithmetic mean, δ is the standard deviation. The statistical processing of 10 data obtained was carried out using the Mann-Whitney test, X2 and the exact Fisher test. The differences were considered statistically significant at p < 0.05.
Sensitivity (Sn) and specificity (Sp) were calculated using the formulas: Sn = a/(a+c); Sp = d/(b+d), where a – true positive results (number of patients with spinal inflammatory pain and diagnosed with axSpA); b – false positives (number of patients with spinal inflammatory pain and not corresponding to the diagnosis of axSpA); c – false negative results (number of patients without dorsal inflammatory pain, but diagnosed with axSpA); d - true negative results (number of patients without dorsal inflammatory pain and without a diagnosis of axSpA).
We also calculated the positive predictive value (PPV) – the probability of having SpA if the patient has spinal inflammatory pain, and the negative predictive value (NPV) – the probability of not having axSpA if the patient does not have spinal inflammatory pain. The probability ratio of positive (LR+) and negative (LR-) results was determined. The higher the LR+, the higher the risk of being diagnosed with SpA if a patient with IID has spinal inflammatory pain. The higher the LR-value, the higher the likelihood of the absence of SpA in the absence of spinal inflammatory pain in a patient with IID.
To determine the odds ratio (OR), a binary logistics regression was built, a multivariate logistics regression model. The calculation was performed using the IBM SPSS 23 statistical program for Microsoft Windows, GraphPad Prism 8.
Results
In patients with IID the most common extraintestinal involvement was of the musculoskeletal system, which occurred in 45 (49.5%) patients. Arthralgia was one of the most common clinical manifestations, occurring in almost a third of patients with IID – 35 (38.5%) patients. Arthritis was detected in 12 (13.2%) patients with IID: in 8 (20.5%) with S±Sh and 4 (7.7%) with Y±C. SpA axial skeletal lesions were present in 26 (28.6%) patients and AS in 14 (15.4%) patients. The incidence of eye injuries was as follows: iridocyclitis – 6 (6.6%) patients, uveitis – 2 (2.2%) patients.
All patients with IID and spinal pain (n = 84) completed the 2009 ASAS questionnaire for spinal inflammatory pain. When evaluating each criterion for spinal inflammatory disease in patients with IID (n = 84), S±Sh (n = 36), Y±C (n = 48) and back pain, it was found that the onset of back pain before the age of 40 years was observed in 63 (75%) patients with IID – 32 (88.6%) patients with S±Sh and 31 (64.6%) with Y±C. The gradual occurrence of back pain was reported by 65 (77.4%) patients with IID – 25 (69.4%) with S±Sh and 40 (83.3%) with Y±C.
Pain relief after effort was present in 53 (63.1%) patients with IID – 24 (66.7%) had S±Sh and 29 (60.4%) had Y±C. Increased pain during rest periods was observed in 42 (50%) patients with IID: 17 (47.2%) with S±Sh, 25 (52.1%) with Y±C. The presence of nighttime back pain (with improvement after waking up) was reported by 52 (61.9%) patients with IID – 20 (55.6%) with S±Sh and 32 (66.7%) with Y±C.
Of all 91 patients with IID, 39 (42.9%) met the criteria for spinal inflammatory disease. Pelvic radiography was performed in 55 patients with IID: all patients with IID (n = 39), as well as patients with IID with suspected spinal inflammatory disease (3 criteria out of 5, n = 16). Changes according to pelvic radiography were detected in 40 patients. Significant SI joints changes were detected in 14 patients.
MRI of the SI joints in T3 mode, was performed in 41 patients with IID and back pain. Bone marrow edema was detected in 6 patients (14.6%), and structural changes were detected in 20 patients with IID (48.7%). It should be noted that at the time of inclusion in the study, the number of patients with AS was 6 (6.6%). According to imaging, SpA was diagnosed in 26 (26.8%) patients – 14 with ankylosing spondylitis and 12 with non-radiological SpA.
It is important to note that in 12 patients with IID, the axial lesion was diagnosed at the early stage. According to the literature, the prevalence of IID-associated SpA varies in the range of 15-37%. The frequency of AS in our study was 15.4%, which is higher compared to the literature data (3-10%) (Table 1).
Table 1. Axial spondyloarthritis in patients with IID | ||
Parameters | IID with SpA (n = 26) | IID without SpA (n = 65) |
| Male (n (%)) | 16 (61.5) 31 (47.7) | 16 (61.5) 31 (47.7) |
Age, years, (M ± δ) | 40.35 ± 10.35 | 40.18 ± 12.36 |
Age at the onset of the disease, years, (M ± δ) | 30.52 ± 11.36 | 32.61 ± 14.06 |
Duration of the disease, years, (n) | 8.23 ± 7.14 | 7.47 ± 7.876 |
Arthralgia, (n (%)) | 16 (61.5) | 19 (29.2)* |
Arthritis, (n (%)) | 9 (34.6) | 3 (4.6)** |
Inflammatory spinal pain (ASAS), (n (%)) | 20 (76.9) | 19 (29.2)** |
Uveitis, (n (%)) | 6 (23.1) | 1 (1.5)** |
Hemoglobin, (M ± δ) | 123.23 ± 23.7 | 124.63 ± 23.61 |
Platelets, (M ± δ) | 349.09 ± 119.1 | 310.73 ± 133.49 |
ESR, (M ± δ) | 25.95 ± 18.66 | 18.24 ± 12.07 |
C-reactive protein, (M ± δ) | 11.78 ± 30.51 | 10.95 ± 25.197 |
y globulins, (M ± δ) | 19.525 ± 4.76 | 18.44 ± 3.80 |
S±Sh, (n (%)) | 16 (61.5) | 23 (35.4)* |
| Activity Y±C | 5.33 ± 1.94 | 5.05 ± 2.45 |
Activity S±Sh | 264.27 ± 159.18 | 225.17 ± 125.92 |
Note: *p < 0.05; **p < 0.01. M – arithmetic mean; δ – standard deviation; IID - intestinal infectious diseases; SpA – spondyloarthritis; ASAS – Assessment of SpondyloArthritis International Society; Y±C - Yersinia enterocolitica or Campylobacter jejuni; S±Sh – Salmonella enteritidis or Shigella flexneri. | ||
The variability of prevalence rates is explained by the heterogeneity of the studies: the incidence of axial joint damage in IID was influenced by the used classification criteria.
Thus, in patients with a combination of IID and SpA, clinical signs of joint damage such as spinal inflammatory pain (p < 0.01), arthralgia (p < 0.05), arthritis (p < 0.01) as well as uveitis (p < 0.01) were encountered significantly more frequently. An analysis of the SpA frequency was made according to the nosology. It has been proven that in patients with S±Sh, axSpA was detected significantly more often (p < 0.05) than in patients with Y±C. When building a logistic regression, it was found that in patients with IID, the risk of detecting SpA was higher in the presence of extraintestinal manifestations - OR 8.07 [95% CI 2.80-23.23], p = 0.0001, arthritis - OR 10.77 [95% 2.26-44.2], p= 0.005, arthralgia - OR 4.12 [95% CI 1.55-10.95], p = 0.005, uveitis - OR 19.2 [95% CI 2.18-169.13].
The activity, duration of the disease and the extent of IID did not increase the risk of axSpA. It was also determined that the risk of having axSpA is higher in patients with S±Sh compared to Y±C - OR 2.92 [95% CI 1.14-7.48], p = 0.025.
Taking into account the findings, in order to verify the reliability of the association of the confusion factor (influence factor) on the detection of axSpA in patients with IID, a multivariate logistics regression model was developed. It turned out that the third model, which included arthritis, S±Sh and spinal inflammatory pain (R2 = 0.486), set the highest rate of correct predictions. Given this, each parameter – spinal inflammatory pain, arthritis, arthralgia and the diagnosis of S±Sh are associated with an increased risk of developing SpA independently of each other, which allows us to state about the applicability of each parameter for the early diagnosis of axSpA in patients with IID.
Lesions of the gastrointestinal tract in ankylosing spondylitis
The analysis of the results of the questionnaire DISQ for the identification of symptoms of gastrointestinal lesions in patients with AS (n = 50) revealed a very heterogeneous frequency of symptoms. The most common symptoms were fatigability – 42 (84%), bloating – 32 (64%) patients, abdominal (or stomach) pain – 33 (66%) patients, and subfebrile condition – 29 (58%) patients.
In addition, the questions were grouped into four blocks depending on the severity of each symptom: the first block included – relation with the frequency of defecation; the second reflected the painful syndrome; the third - dyspepsia; the fourth – the syndrome of asthenia and fever. When calculating the average severity of each symptom, it was found that the complaints predominated in asthenia (2.12 ± 1.29), bloating (1.31 ± 1.19), abdominal pain (1.25 ± 1.17), as well as the subfebrile state.
The average score for the final value of the questionnaire was 9.76 ± 7.73. According to our study, in 46 patients (92%), the score of the results of the questionnaire was less than 19, which was considered remission. Subsequently, patients with a combination of AS and IID (n = 14) were compared with patients with IID-free AS (n = 50).
Thus, patients with a combination of IID and AS were significantly more likely to have signs of eye damage – uveitis and iridocyclitis compared to patients with IID-free AS (p < 0.05). HLA-B27 was detected significantly more frequently in patients with AS than in patients with a combination of IID and AS (p < 0.05).
The diagnostic value of 2009 ASAS classification criteria for spinal inflammatory pain and the possibility of their application to patients with a combination of IID and axSpA has not been determined. In this study, the diagnostic significance of ASAS classification criteria for spinal inflammatory pain was calculated in patients with IID and a concomitant diagnosis of axSpA.
Patients without dorsal pain were excluded from the study (n = 7). Therefore, the number of patients for calculating the diagnostic significance was 84. Patients were separated into two groups according to the presence of IID according to the criteria for spinal inflammatory pain 2009 ASAS classification criteria. The first group consisted of patients with IID and spinal inflammatory pain (n = 39); the second – patients with IID without spinal inflammatory pain (n = 45). Then, in each group, patients were divided into 4 subgroups according to the presence/absence of changes according to imaging data.
Mathematical calculation of the diagnostic significance
The Sn of the spinal inflammatory pain criteria was 75.9%, Sp – 68.3%, PPV – 0.51, NPV – 0.87, LR+ = 2.3, LR - = 0.3. Noteworthy is the high value of NPV – 0.87, that is, in the absence of spinal inflammatory pain, the probability of absence of SpA is 87%.
Therefore, 2009 ASAS classification criteria in IID shows a sensitivity of 75.9% for IID with dorsal inflammatory pain and 77.8% for dorsal inflammatory pain without IID and a specificity of 68.3% for IID with dorsal inflammatory pain, and 74.8% for dorsal inflammatory pain without IID.
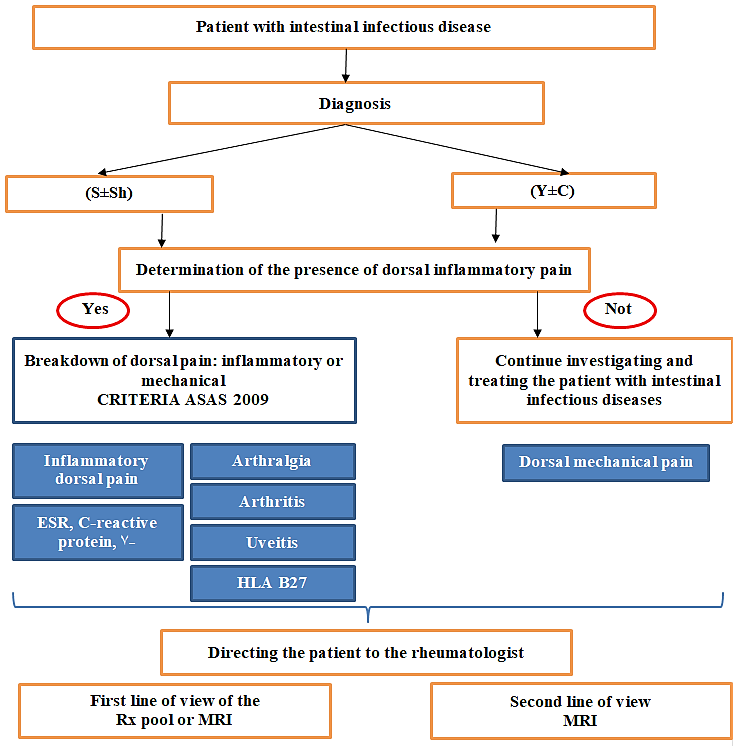
Thus, our study allowed us to establish the following parameters arthritis, arthralgia, spinal inflammatory pain as well as the diagnosis of S±Sh, is raises the suspicion of SpA. It was possible to determine the sensitivity and specificity of the 2009 ASAS classification criteria for spinal inflammatory pain, which demonstrated significantly superior sensitivity and specificity compared to the criteria studied in a cohort of patients with chronic back pain. An algorithm for the early diagnosis of axial arthropathy in patients with IID has been proposed (fig. 1).
|
Fig. 1 Algorithm for diagnosing axSpA in patients with IID ASAS – Assessment of SpondyloArthritis International Society; Y±C – Yersinia enterocolitica or Campylobacter jejuni; S±Sh – Salmonella enteritidis or Shigella flexneri; MRI – Magnetic Resonance Imaging; Rx – Radiography; HLA – Human Leukocyte Antigens; ESR – Erythrocyte Sedimentation Rate. |
Conclusions
In patients with IID, the following clinical variants of arthropathies have been identified: axial lesion (spinal inflammatory pain according to 2009 ASAS classification criteria in 42.9%, axSpA in 28.6%, AS in 15.4%); arthralgia – 38.5%, arthritis – 13.2%. Conventional radiography imaging and MRI of SI joints increased the incidence of SpA from 6.6% (n = 6) to 28.6% (n = 26).
In patients with IID and axial arthropathies, arthralgia (p < 0.01), arthritis (p < 0.01), and uveitis (p < 0.01) are more common. At the same time, the possibility of detecting axSpA is higher in the presence of arthritis - OR 10.77 [95% CI 2.26-44.2], p = 0.005, arthralgia - OR 4.12 [95% CI 1.55-10.95], p = 0.005, inflammatory back pain - OR 8.07 [95% CI 2.80-23.23], p= 0.0001, uveitis - OR 19.2 [95% CI 2.18-169.13], p = 0.008. Patients with S±Sh have a higher chance of developing axSpA compared to patients with Y±C - OR 2.92 [95% CI 1.14–7.48], p = 0.025.
In patients with AS, according to the results of the questionnaire for the identification of gastrointestinal symptoms DISQ, complaints of asthenia prevailed, an average score of 2.12 (moderately pronounced), bloating and abdominal pain (an average score of 1.31 and 1.25, respectively), subfebrile condition (average score 1.08). At the same time, the lesion of the gastrointestinal tract was subclinical, since, according to the total score, 92% of the patients corresponded to the remission criteria.
The 2009 ASAS classification criteria for the evaluation of spinal inflammatory pain are applicable for patients with IID: sensitivity – 75.9%, specificity – 68.3%, positive predictive value – 0.51, negative predictive value – 0.87, the ratio of the probability of a positive result - 2.3, the probability ratio of a negative result - 0.3; for S+Sh: Sn = 71.4%, Sp = 72.7%, PPV = 0.63, NPV = 0.8, LR+ = 2.62, LR- = 0.39; for Y±C : Sn = 83.3%, Sp = 63.9%, PPV = 0.44, NPV = 0.92, LR+ = 2.31, LR- = 0.26.
An algorithm was proposed for the early detection of axial arthropathy in patients with IID with a certain diagnostic value of the ASAS classification criteria and an OR for spinal inflammatory pain, arthritis, arthralgia, uveitis, and the diagnosis of S±Sh.
Abbreviations
ASAS – Assessment of SpondyloArthritis International Society; IID – Intestinal Infectious Diseases; MRI – Magnetic resonance imaging; Rx – X-ray; S±Sh – Salmonella enteritidis or Shigella flexneri; SpA – Spondyloarthritis; Y±C – Yersinia enterocolitica or Campylobacter jejuni.
Competing interests
None declared
Authors' contribution
Study conception and design: LC, LG. Data acquisition: LC, ER, AS. Analysis and interpretation of data: LC, ER. Drafting of the manuscript: LC. Significant manuscript review with significant intellectual involvement: LC, ER. Approval of the "ready for print" version of the manuscript: LC, ER, LG, AS.
Authors’ ORCID IDs
Lia Chișlari - https://orcid.org/0000-0002-7088-568X
Liliana Groppa - https://orcid.org/0000-0002-3097-6181
Eugeniu Russu - https://orcid.org/0000-0001-8957-8471
Svetlana Agachi - https://orcid.org/0000-0002-2569-7188
References
Pedersen S., Maksymowych W. The Pathogenesis of Ankylosing Spondylitis: an Update. Curr Rheumatol Rep 21, 58 (2019).
Harkins P., Burke E., Swales C., Silman A. ‘All disease begins in the gut’—the role of the intestinal microbiome in ankylosing spondylitis, Rheumatology Advances in Practice, Volume 5, Issue 3, 2021.
Breban M., Beaufrère M., Glatigny. The microbiome in spondyloarthritis. Best Practice & Research Clinical Rheumatology 33.6 (2019): p.101495.
Fragoulis C., George E., et al. Intestinal infectious diseases and spondyloarthropathies: from pathogenesis to treatment. World journal of gastroenterology 25.18 (2019): p.2162.
Belousova E., Abdulganieva D., Odintsova A. Frequency of Inflammatory Back Pain and Structural Changes of Axial Skeleton on Inflammatory Bowel Disease. Book of abstracts. 2nd Prague European Days of Internal Medicine. Prague, 2016, № 1-2., p. 8.
Belousova E., Abdulganieva D., Odintsova A. Performance of ASAS criteria for inflammatory back pain in patients with inflammatory bowel disease. Journal of Crohn’s &Сolitis. 2017. Vol. 11 (February), p. 56.
Ahsan T., Erum U., Jabeen R., Khowaja D. Ankylosing Spondylitis: A rheumatology clinic experience. Pak J Med Sci. 2016;32(2), p.365-368.
Young A., Lianjun A W., Liping A W., Xin A X., Cory J. A L., Hai D A. Possible Role of Intestinal Microbiota in the Pathogenesis of Ankylosing Spondylitis. 2016 J International Journal of Molecular Sciences, V 17 N 12, p.2126.
Rocha F., Castro A., et al. Microbes, helminths, and rheumatic diseases. Best Practice & Research Clinical Rheumatology 34.4 (2020): p.101528.
Tingting W., Shuhui M., Ping C., Laiyou W., Cuilian L., Donge T., Dongzhou L., Zhenyou J., Xiaoping H. Comprehensive analysis of differentially expressed mRNA and circRNA in Ankylosing spondylitis patients’ platelets. Experimental Cell Research, (2021).
Antoniou A., Lenart I., Kriston-Vizi J., et al. Salmonella exploits HLA-B27 and host unfolded protein responses to promote intracellular replication. Annals of the Rheumatic Diseases 2019;78, p.74-82.
Kurtz J., Goggins J., McLachlan J. Salmonella infection: Interplay between the bacteria and host immune system. Immunol Lett. 2017;190: p.42-50.
Ashrafi M., Kuhn K., Weisman M. The arthritis connection to inflammatory bowel disease (IID): why has it taken so long to understand it?. RMD Open 2021; p.7.
Cardoneanu A., et al. Characteristics of the intestinal microbiome in ankylosing spondylitis. Experimental and therapeutic medicine 22.1 (2021): p.1-14.
Zhang X., Sun Z., Zhou A., et al. Association Between Infections and Risk of Ankylosing Spondylitis: A Systematic Review and Meta-Analysis. Front Immunol. 2021;12:768741. Published 2021 Oct 22.