Introduction
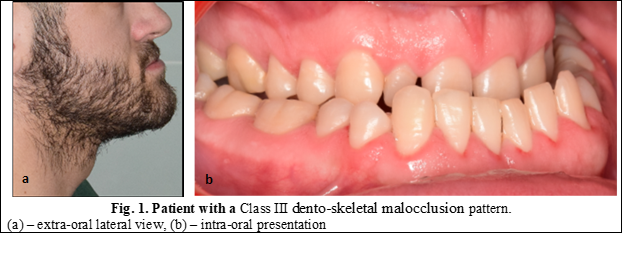
According to Moss’s functional matrix theory, bone growth occurs in response to function, thus, tongue posture, nasal resistance and the presence of adenoids are thought to affect maxillofacial form [1, 2]. Class III dento-skeletal anomalies or mandibular prognathism is a facial disharmony characterized by an anterior relationship of the mandible to the upper jaw [3]. These changes in the sagittal plane of the upper jaw compared to the lower one translates into facial analysis through a face with a concave profile, sunken cheeks, the upper lip usually being placed in the opposite direction to the lower lip (Figure 1). These features lead to significant physiological and functional disorders with impaired quality of life [4]. The etiology of this anomaly has not yet been fully elucidated, but most authors assume that the occurrence of this disease may be conditioned by specific genetic factors, environment, and their relations [5].
The specific factors are characterized by the presence of disorders during embryological development, discrepancies in skeletal growth and / or dental arches, pituitary dwarfism, acromegaly but also early tooth loss. Genetic factors refer to the transmission of family tendencies, such as the disproportion between the size of the teeth and jaw bones which could generate crowding or spacing, as well as the disproportion between the size and shape of the upper jaw to the lower [6]. Environmental factors are the disruption of the balance of harmonious development due to the influence of external pressure [6]. Such pressures can be caused by the interposition of objects between the dental arches, such as the lower or upper lip, fingers, extended use of the pacifier, infant swallowing, mouth breathing, etc. Similarly, authors such as Brodie et al., have assumed that tongue volume (VL), in addition to posture and function, is of notorious importance in the development of dento-facial anomalies [7].
Currently in the scientific literature, it is debated whether the tongue actively shapes the surrounding tissues or adapts to existing conditions. Also, the clinical studies started by Tamari et al. and Yoo et al., concluded that the volume of the tongue is correlated with the size of the lower dental arch [8], face height and chin position [9]. Previous research has also shown a correlation between the lower position of the tongue and the narrowing of the upper jaw in association with the posterior cross-bite, while infant swallowing is associated with open bite. Thus, lately, more and more authors show the importance of determining the volume of the tongue, because an increased volume will lead to a decrease in the total volume of the oral cavity. Consequently, by performing only mandibular setbacks, relapse may occur, therefore authors such as Clauser and Tieghi, in case of macroglossia, consider the surgical reduction of the tongue [10]. Studies on the surgical reduction of tongue at patients with Beckwith-Wiedmann syndrome in preadolescence have shown a change in skeletal class from III to I [10], but it was also associated with a lingual collapse of the dental arches [11]. For this reason, to better understand the influence of tongue on occlusion stability after orthognathic surgery, it is important to evaluate the oral cavity volume (OCV) and tongue volume (TV) to determine the volume ratio between TV and OCV.
The aim of this study is to evaluate the volume of the tongue in patients with class III malocclusion compared to class I patients, using three-dimensional volumetric analysis based on computed tomography. Thus, the null hypothesis assumes that there is no correlation between the volume of the tongue and the maxillo-mandibular relations in the third skeletal class patients.
Materials and methods
The current retrospective study involved 15 patients who came for dental treatment during 2019-2021. Patients were divided into 2 groups depending on their malocclusion type, thus the study group included 10 patients with class III, who had a mesialized molar ratio and a negative overjet, of which 3 women and 7 men (mean age – 29.8 years ± 8.25); and the control group included 5 patients with orthognathic occlusion, the relationship of class I molars with an overjet and overbite within the normal range, of which 2 women and 3 men (mean age – 36, 6 years ± 13.59).
The inclusion criteria were the presence of computed tomography (to minimize radiation exposure, the study also included class I patients who underwent radiological examination for purposes other than orthodontic examination, such as various traumas in the middle part region and upper face, tumors of the soft tissues of the face, etc.).
Exclusion criteria included: the presence of orthodontic treatment in the anamnesis, craniofacial developmental abnormalities, traumas in the region of the lower third of the face as well as disorders of the temporomandibular joint.
The study was approved by the Ethics Committee of the N. Testemițanu State University of Medicine and Pharmacy, no.23 from 12.04.2019 and informed consents were obtained from all subjects involved in the study.
During the radiological examination, the patient was placed in a supine position in the Siemens -Somatotom Definition Edge tomography device, with the occlusal plane oriented perpendicular to the tube (gantry), slice thickness 0.5 mm, field of view 218 mm, gantry tilt 00, at 120kV and 209 mA, for 12.4 seconds. Each radiological study included 523-612 native slices exported in DICOM format, the size of a voxel being 0.5 mm.
Applying the three-dimensional cephalometric analysis protocol, the maxilla-mandibular ratio in the sagittal plan was evaluated, using the angles: sella-nasion-A (SNA), SNB as well as FMA (Frankfort plan angle to the mandibular plan). The anatomical points were drawn manually in each patient.
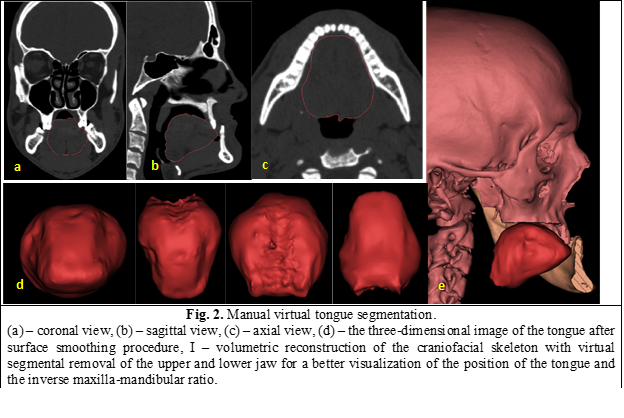
The evaluation of the tongue volume was performed in the open-source software Slicer 3D 4.10.1 (https://www.slicer.org/), using the manual virtual segmentation procedure, based on the density range specific to muscle tissue, from -200 to 200 HU (Hounsfield units). In the segmented volume (Figure 2) were included 3 intrinsic muscles (longitudinal, transverse, vertical), 3 extrinsic muscles (genioglossus, palatoglossal, styloglossus). Hyoglossus muscle was not included in the segmentation.
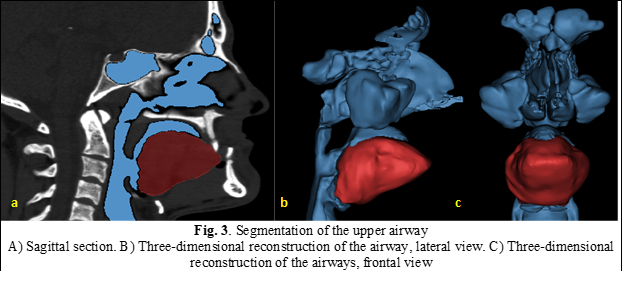
To reduce segmentation errors, upper airway segmentation was additionally performed, and the Boolean subtraction procedure was carried out (Figure 3).
In this study, the t-Student test was performed to assess the volume of tongue in males and females, but also to compare the study groups with each other, in the IBM SPSS statistical program. To evaluate the relationship between the volume of the tongue and the prognathism of the mandible the Pearson correlation was calculated (Class III Angle). Results are considered to be statistically significant at a level lower than 5% (p <0.05). Data distribution assumption of normality was respected.
Results
The average volume of the tongue in this study was 74.55 cm3, in class I – 65.35 cm3 and in class III – 79.15 cm3 (Figure 4). The data (mean and standard deviation) are represented in Table 1.
Table.1. Statistical comparison of study groups | ||||
| Class I | Class III | ||
average | stand.dev. | average | stand.dev. | |
Age | 36.60 | 13.594 | 29.89 | 8.257 |
SNA | 82.20 | 2.588 | 81.20 | 3.364 |
SNB | 80.80 | 3.962 | 87.75 | 5.195 |
ANB | 2.20 | 0.836 | -7.23 | 5.137 |
FMA | 23.02 | 2.331 | 22.75 | 3.955 |
Witts | 1.38 | 0.704 | -12.19 | 6.469 |
Tongue volume in cm3 | 65.89 | 11.645 | 79.15 | 17.612 |
Airway volume in cm3 | 96.48 | 33.964 | 114.15 | 30.117 |
Note: SNA-the angle between the sella/nasion plane and the nasion/A plane; SNB-the angle between the sella/nasion plane and the nasion/B plane; ANB-difference between SNA and SNB angles; FMA-the angle formed by the intersection of the Frankfort horizontal plane with the mandibular plane; Witts-appraisal entails projecting points A and B in two perpendicular lines, along the functional occlusal plane. | ||||
Given the obvious sexual dimorphism, there was also conducted a comparison of the tongue volume, depending on gender (Figure 4).
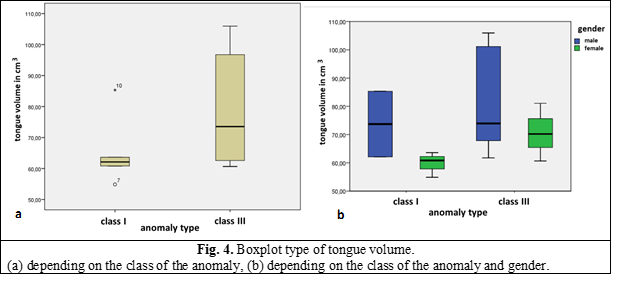
The Pearson correlation revealed a positive and statistically significant correlation between the tongue volume and the SNB angle value, P = .034 (if the tongue volume is larger, the SNB angle increases) and negatively correlated with the Wits value P = .005 (if the tongue volume increases, Wit value decreases). The volume of the airways was 108.26 cm3.
Discussions
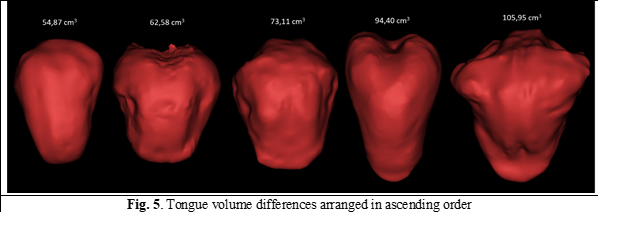
In 1965, Köle assumed that macroglossia could be the cause of the development of mandibular prognathism but failed to elucidate the role of language in the development of dento-maxillary anomalies [12]. This is due to the lack at that stage of the possibilities of objective quantitative and qualitative calculation of the volume of the language. The present study showed that the volume of the tongue was higher in patients with Class III compared to Class I (Figure 5).
Similar results were noticed in the study conducted by Ihan et al., in 2016, where the volume of the tongue in males was 100.8 ± 6.3 cm3 in class III compared to 92.4 ± 9.8 cm3 in class I, and in females – 77.4 ± 10.2 cm3 in class III compared to 67.2 ± 5.6 cm3 in the control group [12]. In another study conducted by Shigeta et al., in Japan on a group of 40 patients, the mean volume of the tongue was 79.00 ± 1.06 cm3, the age of the subjects was between 25 and 77 years, however, in this study, no segmentation limits were defined and an automatic and/or semi-automatic segmentation protocol was used [13]. Similar results have been described by other authors, the results varying between 30 to 132 cm3, these discrepancies are represented by the measurement method (direct measurements, use of different impression techniques), acquisition technique (multidetector computed tomography, conical beam computed tomography, magnetic resonance, ultrasonography) but also by the boundaries that the authors used in estimating the volume of the tongue. Some studies also included the pharyngeal muscles, while in other publications the volume of the tongue was partially calculated, where the posterior nasal spine was used as a posterior landmark and the lower border was the enamel-cement junction of the first lower molars. For this reason, in some research there is a correlation between the volume of the tongue and the SNB angle values as well as the narrowing of the upper jaw, similar results being obtained by Iwasaki et al., in 2019. Based on the data presented, treatment planning should be performed with caution, considering the volume of the tongue, because in certain clinical situations, some authors recommend performing dental extractions to align and camouflage the dento-facial anomaly. However, due to the decrease in the perimeter of the dental arches, the pressure exerted by the tongue could favor the development of relapse. At the same time, the planning of orthognathic surgery must also be performed depending on the volume of the tongue and the oral cavity, to achieve a volumetric balance between them. Currently we cannot correlate the volume of the tongue with the pressure it exerts, because we do not know much about the self-adaptive abilities of the tongue and its changes in tonicity. Interestingly, in a study conducted by Frohlich et al., the pressure of the tongue on the teeth after surgical reduction did not change significantly at 12 months postoperatively, but the resting pressure was lower than before surgery [14]. For these reasons, in cases of increased tongue pressure following the posterior movement of the mandible, Wickwire et al., recommended reducing the tongue to achieve better stability of the intervention [15]. Still, such an approach is quite traumatic not only in our opinion but also in other authors’ as well, because it can involve several postoperative complications. In such cases, we consider the bimaxillary approach to be rational, because the advancement of the upper jaw reduces the posterior movement of the mandible, so we do not adversely affect the volume of the airways.
In this study, the volume of the tongue was not correlated with the position of the hyoid bone; this remains to be elucidated in future research. Based on the obtained results, we are also going to study how the volume of the oral cavity changes after performing orthognathic surgery as well as its influence in the development of sleep apnea.
Conclusions
Based on the limitations of the current study, we can conclude that the increased volume of the tongue is correlated with mandibular prognathism. This phenomenon was encountered in both sexes, as well as the increased volume was correlated with the pronounced negative Witts values. The clinical significance of the present study is that the posterior movement of the mandible in orthognathic surgery should be carefully planned to minimize the risk of relapse as well as narrowing of the airways.
Competing interests
None declared
Financial disclosure statement
The research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author’s ORCID ID
Stanislav Strîșca - https://orcid.org/0000-0002-6215-9434
References
Iwasaki T., Sato H. et al. Relationships among nasal resistance, adenoids, tonsils, and tongue posture and maxillofacial form in Class II and Class III children. Am J Orthod Dentofacial Orthop. 2017;151(5):929-940.
Iwasaki T., Suga H, et al. Relationships among tongue volume, hyoid position, airway volume and maxillofacial form in paediatric patients with Class-I, Class-II and Class-III malocclusions. Orthod Craniofac Res. 2019;22(1):9-15.
Trifan V., Lupan I. et al. Morbiditatea prin anomaliile dento-maxilare în Republica Moldova. Medicina Stomatologică. 2015;1(34):47.
Mendes de P. G., Adas S.G. et al. Dentofacial Deformities and Implications on Quality of Life: A Presurgical Multifactorial Analysis in Patients Seeking Orthognathic Surgical Treatment. J Oral Maxillofac Surg. 2019; 77(2):409.e1-409.
Mi H. et al. Influence of sociocultural factors on the selection of orthognathic surgery in patients with dental and maxillofacial deformities. Shanghai Kou Qiang Yi Xue. 2018;27(5):495-500.
Gabardo M., Zielak J. et al. Impact of orthognathic surgery on quality of life: Predisposing clinical and genetic factors. J Craniomaxillofac Surg. 2019;47(8):1285-1291.
Brodie A.G. Muscular factors in the diagnosis and treatment of malocclusions. Angle Orthod. 1953;23(2):71–7.
Tamari K., Shimizu K. et al. Relationship between tongue volume and lower dental arch sizes. Am J Orthod Dentofacial Orthop. 1991;100(5):453-8.
Yoo E., Murakami S., Takada K. et al. Tongue volume in human female adults with mandibular prognathism. J Dent Res. 1996;75(12):1957-62.
Miyawaki S. et al. Long-term changes in dentoskeletal pattern in a case with Beckwith-Wiedemann syndrome following tongue reduction and orthodontic treatment. Angle Orthod. 2000;70(4):326-31.
Liu Z.J., Shcherbatyy V. et al. Effects of tongue volume reduction on craniofacial growth: A longitudinal study on orofacial skeletons and dental arches. Arch Oral Biol. 2008;53(10):991-1001.
Ihan H. N., Barbič U. Tongue volume in adults with skeletal Class III dentofacial deformities. Head Face Med. 2016;22;12:12.
Shigeta Y., Ogawa T. et al. Influence of tongue/mandible volume ratio on oropharyngeal airway in Japanese male patients with obstructive sleep apnea. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(2):239-43.
Frohlich K., Ingervall B., Schmoker R. Influence of surgical tongue reduction on pressure from the tongue on the teeth. Angle Orthod. 1993;63(3):1918.
Wickwire N.A., Proffit W.R. Changes in tongue position and activity following mandibular osteotomy. Am J Orthod. 1972;62(1):94–5.).