Introduction
The modern pathogenetic model of atopic dermatitis (AD) can be represented as a chain, the links of which are considered:a set of predisposition genes, a reduced barrier function of the skin, a disorders of innate immunity, which are joined by external factors and characteristics of the adaptive immune response.The most significant genetically determined disorders in this disease are changes in the immune system and the skin barrier, the dysfunction of which is a favorable background for the development of AD [1, 2].
Most experts support the concept of AD development as a disease associated with abnormalities of the epidermal barrier [3, 4].
The increased activity of own proteases, which destroy structural components in the epidermis, and the presence of additional exogenous proteases produced, for example, by house dust mites or Staphylococcus aureus, facilitate the destruction of desmosomal contacts and the penetration of allergens into the skin. The functioning of the epidermal barrier is also affected by a disorder in the maturation and advancement of lamellar granules, which leads to a significant deficiency of acid, lipid, and enzyme components of the stratum corneum. AD-associated increase in the activity of sphingomyelin deacylase leads to a decrease in the production of ceramides [5, 6].
Damaged skin barrier is the most important factor in AD development, which is indirectly confirmed by the fact that the most characteristic places of localization of lesions in this disease are areas of the skin with a weakened epidermal barrier and increased protease activity [5, 6]. Constitutionally dry skin is also considered an essential feature, which is due to the reduced level of ceramides, squalene and free fatty acids in the stratum corneum lipidscomponents of the natural moisturizing factor, the appearance of defects in intercellular lipid layers, increased transepidermal water loss [7].
Topical steroids, which are the main means of external treatment of patients with chronic dermatoses, depending on the dosage form and the strength of the corticosteroid, to one degree or another deepen atrophy, dryness, dehydration of the skin. The third generation of corticosteroids includes a large number of fluorinated glucocorticosteroids that have a strong and very strong local effect. These agents have more favorable properties of pharmacokinetics.At the same time, with long-term use of steroids, hypopigmentation, secondary infection, acne and striae, an increase in the level of cortisol in the blood, and aggravation of the course of osteoporosis are more common.Their development is due to the inhibition of fibroblast proliferation and collagen synthesis, as well as inhibition of the proliferative activity of keratinocytes and fibroblasts. In this regard, external remedies for AD patients, especially those with a filaggrin gene mutation, need appropriate correction to prevent the deepening of the morphological and functional changes inherent in this condition [8, 9]. The corresponding correction can be carried out by including ceramides in the base of the drug, which contribute to the restoration of the epidermal barrier due to the filling of lipids and are a conductor of active substances into the dermis [10].
The aim of the work was to justify the inclusion of ceramides in the base of topical fluorinated steroid drugs for the treatment of allergic dermatoses by studying the morphological changes in the skin of guinea pigs after applying the above-mentioned products of various compositions.
Materials and methods
The study was conducted on outbred guinea pigs of the same sex and fatness (40 individuals). The animals were divided into separate groups depending on the applied drug. The animals were on a standard diet and received food that was equivalent in terms of qualitative and quantitative composition. The conditions of the animals were in accordance with the rules of the "European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes".
The possibility of correcting the complications of topical steroid use (development of skin atrophy, increased xerosis, etc.) was studied in an experiment by introducing ceramides into the base of topical fluorinated steroids.
The following combinations of drugs and, accordingly, guinea pigs were selected, which were divided as follows: IC – intact control (15 animals); O – base application (14); OC – base containing ceramides (15); FSt/kr – fluorinated steroids of strong action (betamethasone) (16); FStK/kr – fluorinated steroids of strong action (betamethasone) + ceramides (14).
During the external examination, clinical changes were assessed on the excised areas of the skin, where samples were subsequently taken for histological examination. The samples were fixed in a 10% solution of neutral formalin, processed in alcohols of increasing concentration (for at least 3 weeks), embedded in celloidin paraffin. Sections with a thickness of 6–7 μm were stained with hematoxylin and eosin. The light-optical research was carried out under a Biolam R-12 microscope. The areas that were most typical for this experimental group were selected for the photos.
Statistical processing of the obtained results was carried out using a package of application programs for Microsoft Excel 2003. Analysis of qualitative data was carried out using the χ2 test. Average arithmetic values for a series of data (M) and errors of average values (m) were calculated. The reliability of the obtained data was assessed by pairwise comparison and determination of the confidence interval based on the calculation of the Student coefficient (t).Differences were considered statistically significant at p<0.05 [11].
Results and discussion
In order to study the traumatic effect of topical steroids on the skin and the possibility of preventing morphological (atrophic) skin disorders by varying the components of an external agent: both the active substance (corticosteroid) and components that reduce their harmful effect (ceramides), an experimental study of the morphology of the skin of guinea pigs was carried out on a model of atrophy caused by long-term (14 days) application of topical fluorinated steroids of various compositions.
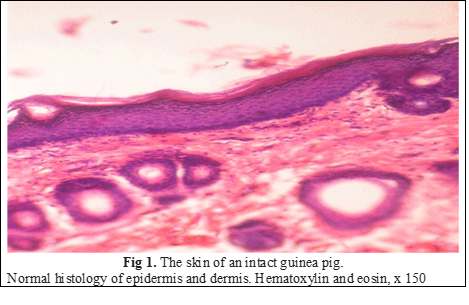
When examining all micropreparations of the IC group, a clear division into the epidermis and the skin itself, which had a normal structure, was revealed. In the multilayered epidermis, all the transitions from viable dividing cells to those that were keratinized took place. The basal layer consisted more often of vertically elongated keratinocytes with dark nuclei, the outlines of which were clear, mitoses were few. The spinous layer had several rows (4–6) of sufficiently densely arranged polygonal cells. As a rule, intercellular contacts were clearly visible. The epidermocytes of the granular layer (3–4 rows) are polymorphic, containing a large number of keratohyalin granules in the cytoplasm, from dispersed in the lower rows to large sharply basophilic closer to the stratum corneum. The thickness of the stratum corneum was uniform throughout the section. Its compact layer tightly adhered to the surface of the epidermal layer, loose, moderately desquamated from its surface. The cellular elements of different layers of the dermis are typical, all obligate derivatives are present, the vessels of the microcirculatory channel had a normal structure. Endothelial cells contained flat, sharply basophilic nuclei. The blood vessel filling was moderate (Fig. 1), the width of the epidermis on average for the group was 15.3 u.o.
The thickness of the epidermal layer of guinea pigs of the studied groups is shown in the table 1.
Table 1. The thickness of the epidermal layer of guinea pigs (u.о.) | |
Group | The thickness of the epidermal layer |
ІC, n = 15 | 15,3 ± 0,91 |
О, n = 14 | 20,75 ± 1,78 |
ОC, n = 15 | 34,71 ± 3,07*† |
FSt/kr, n = 16 | 10,25 ± 0,81* |
FStK/kr, n = 14 | 20,14 ± 1,47† |
Note. * – significantly differs in relation to IC (р < 0,05); † – is significantly different in relation to a similar drug that does not contain ceramides (р < 0,05). | |
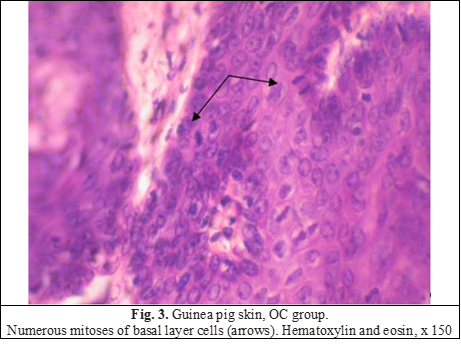
Applying the foundation to animals led to some thickening of the epidermal layer, the morphology of the epidermis and the skin itself was not disturbed at the same time. Thickening occurred due to an increase in the number of rows of the spinous layer (up to 8-9 rows), the exfoliation of the stratum corneum increased (Fig. 2a). The addition of ceramides led to the acceleration of proliferative processes and the development of proliferative acanthosis (Fig. 2b). Mitotically dividing cells were found more often than in intact animals (Fig. 3), increased epidermopoiesis led to a pronounced thickening of the epidermal layer due to the spiny layer, which was significantly thickened (up to 15-16 rows). In some areas, the epidermis formed acanthous outgrowths that penetrated the dermis, and there were horn cysts. On average, the thickness of the epidermal layer in the group exceeded the control indicators by 67% (Table 1). Dystrophic and degenerative phenomena were not observed.
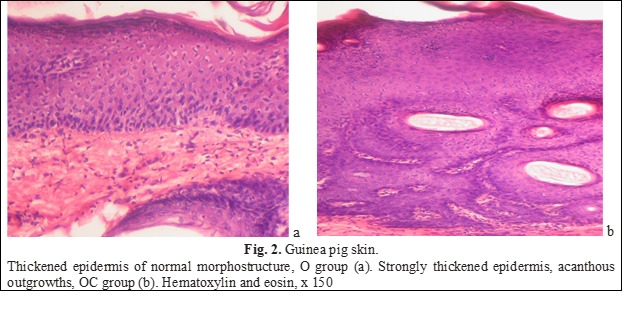
Changes induced by betamethasone (group FSt/kr), were observed mainly in the epidermis.First of all, local atrophy of the epidermal layer should be noted. Thinning was uneven, mostly interfollicular (located between two hair follicles) areas of the skin were altered (Fig. 4a), although occasionally there was atrophied epidermis located above the follicles (Fig. 4b).
On average, the thickness of the epidermal layer was reduced by a third compared to the control group (Table 1). If we measure the thickness of the epithelial layer only in places of thinning, it was 6.17 u.o, which is only 40% of the control values. Atrophic epidermis contained from 3 to 6 rows of keratinocytes.
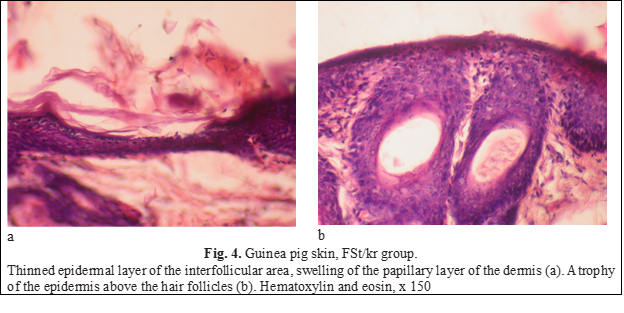
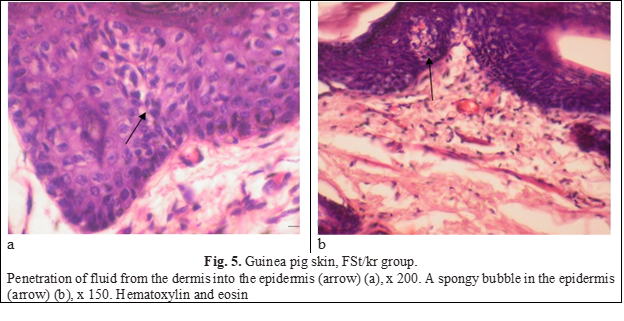
As is known, the development of atrophy is preceded by an edematous-inflammatory stage, the signs of which were found in the skin of animals of this group. Pronounced swellings were noted in the papillary layer of the dermis (Fig. 4а). Fluid from the dermis penetrated the epidermis, expanding and breaking intercellular connections and forming spongy bubbles (Fig. 5).
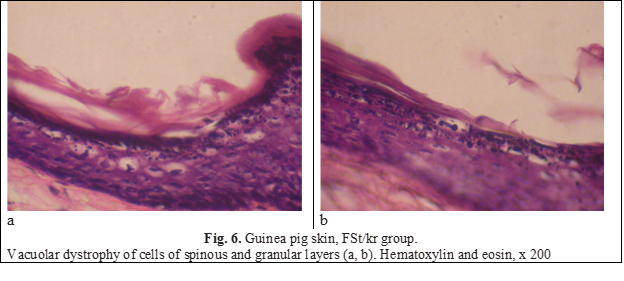
In parallel, dystrophic manifestations developed - intense intracellular swelling of keratinocytes of the spinous and granular layers was noted (Fig. 6).
Vacuoles were located perinuclearly, and in more pronounced cases occupied the entire cytoplasm, shifting the nucleus to the periphery and making it flatter. Damage to keratinocytes and loss of intercellular connections led to acantholysis. Acantholytic spherical cells with oxyphilic cytoplasm were located, as it were, separately from the surrounding cells and were found along the entire length of the epidermis (Figure 7).
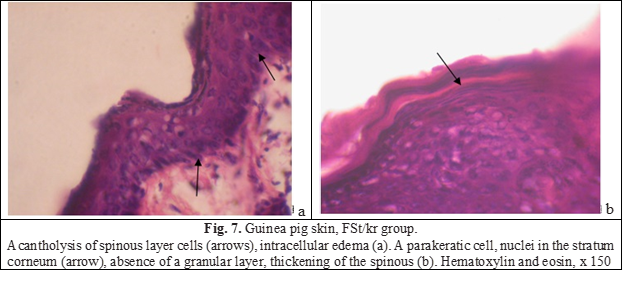
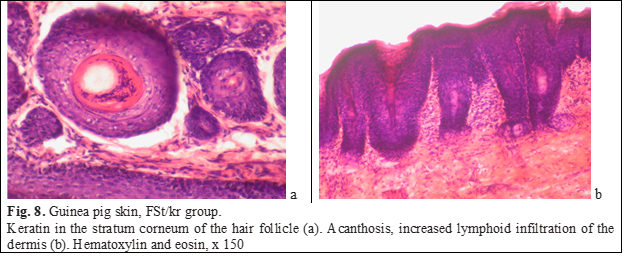
The process of keratinization was also disturbed: there were many intraepidermal horn cysts filled with horn masses, and rare foci of parakeratosis were also identified. The stratum corneum cells in the parakeratic cells appeared immature because they contained rod-shaped, horizontally oriented nuclei (Fig. 7b). The stratum corneum outside the foci of parakeratosis is usually partially or completely exfoliated. The granular layer between the skin layer is expressed unevenly, under areas of parakeratosis, as a rule, it is absent, the spiky layer is thickened. The stratum corneum, which contained an excess of keratin, was also determined in hair follicles (Fig. 8а). Cellular infiltration of the papillary layer of the dermis was enhanced, lymphocytes predominated in the infiltrate (Fig. 8b). The blood supply of the vessels of the dermis was reduced.
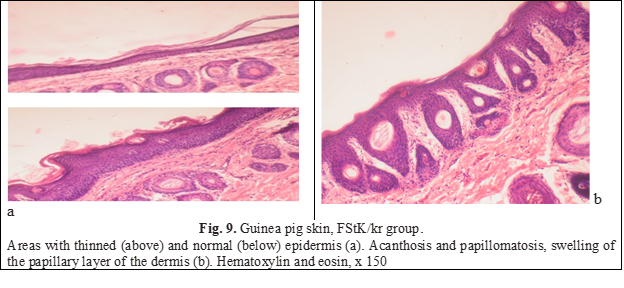
The addition of ceramides to a potent fluorinated steroid to some extent smoothed out the negative effects of the corticosteroid hormone. The average thickness of the epithelial layer increased by 2 times compared to the thickness of the epithelial layer of animals of the FSt/kr group and even slightly exceeded the indicators of intact animals (Table 1). It should be noted the unevenness of the epidermis: areas of normal thickness alternated with thin (rarely) or thickened ones (Figure 9а). Mitoses in the basal layer were more frequent than in the previous group. Acanthosis was the result of the formation of long epithelial outgrowths that deeply penetrated the dermis. In response to the elongation of the epidermal appendages, the dermis reacted with swelling hypertrophy of papillae that penetrated deeply into the epidermis and elongation of capillaries, that is, papillomatosis (Fig. 9b).
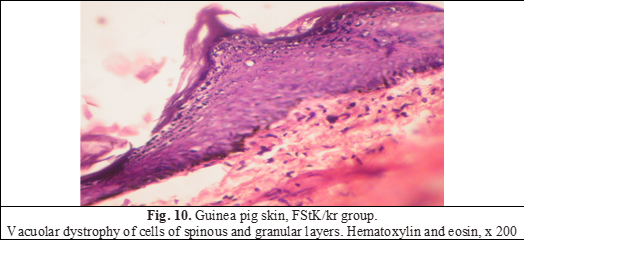
Vacuolar dystrophy of cells of spinous and granular layers was found in practically all animals (FIg. 10). Lymphoid infiltration of the dermis, parakeratosis and acantholysis were not noted.
Thus, the investigated fluorinated corticosteroid (betamethasone) led to the development of skin atrophy in experimental animals, the epidermis of which was thinned by 33% compared to the IC group).
Thinning of the skin in all animals occurred due to the thinning of the epidermal layer, and not the skin itself. Atrophy was accompanied by the development of dystrophic processes of a non-specific nature both in the epidermis and in the dermis.
Ceramides, when added to the studied drugs, had a selective effect: the more significant the atrophy induced by corticosteroids, the more noticeably ceramides enhanced the proliferation of keratinocytes and the stronger the epidermis thickened not only relative to the positive ((by 96%), but also IC (by 32%). If the thinning was insignificant, then the effect of adding ceramides was not so indicative.
As for the application of the base, to which the skin of animals reacts by thickening, ceramides potentiate this property of the base and cause further significant thickening of the epidermis. Ceramides also significantly reduce the manifestations of inflammatory and dystrophic processes in the skin.
Thus, for the external treatment of chronic dermatoses in patients with a mutation of the filaggrin gene, that is, before treatment, they already have skin changes characteristic of these conditions (atrophy, dryness, dehydration, desquamation, etc.), it is advisable to use topical steroids with the addition of ceramides. The development of this class of drugs will prevent side effects that are caused or exacerbated by topical steroids
Conclusions
The inclusion of ceramides in a topical fluorinated corticosteroid (betamethasone) prevented the traumatic effect of hormones by increasing the proliferation of keratinocytes and thickening the epidermis. The use of these means will prevent the occurrence or aggravation of side effects.
Competing interests
None declared.
Authors' contributions
All the authors have contributed equally at the results presentation in the paper.
Authors’ ORCID IDs
Yanina Kutasevych - https://orcid.org/0000-0001-8706-1487
Mykola Lyapunov - https://orcid.org/0000-0002-5036-8255
Iryna Ziuban - https://orcid.org/0000-0003-1323-6509
Iryna Mashtakova - https://orcid.org/0000-0002-3592-6896
References
Tamrazova OB, Gureeva MA, Kuznetsova TA, Vorob'eva AS. Vozrastnaia evoliutsionnaia dinamika atopicheskogo dermatita [Age evolutionary dynamics of atopic dermatitis]. Pediatriia. Zhurnal im. G.N. Speranskogo. 2016;95(2):153-159. Russian.
Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(1):8-16. doi: 10.1159/000370220.
Gorlanov IA, Leina LM, Miliavskaia IR., Kulikova SIu. O zabolevaniiakh, assotsiirovannykh s narusheniem bar'ernoi funktsii kozhi [About diseases associated with impaired skin barrier function]. Pediatr. 2013;4(3):111-114. Russian.
Kim KH. Overview of atopic dermatitis. Asia Pac Allergy. 2013;3(2):79-87. doi: 10.5415/apallergy.2013.3.2.79.
Ionesku MA. Kozhnyi bar'er: strukturnye i immunnye izmeneniia pri rasprostranennykh bolezniakh kozhi [Skin barrier: structural and immune changes in common skin diseases]. Ross Allergol Zh. 2014;(2):83-89. Russian.
Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22(2):125-137. doi: 10.5021/ad.2010.22.2.125.
Tamrazova OB, Molochkov AV. Kseroz kozhi – osnovnoi patogeneticheskii faktor razvitiia atopicheskogo dermatita [Skin xerosis is the main pathogenetic factor in the development of atopic dermatitis]. Dermatologiia. 2014;(4):48-54. Russian.
Mal'chenko EE, Nemchaninova OB, Maksimov VN. Rol' filaggrina v razvitii khronicheskikh zabolevanii kozhi. Obzor literatury [The role of filaggrin in the development of chronic skin diseases. Literature review]. J Siberian Med Sci. 2015;(3):28-34. Russian.
Arkwright P, Motala C, Subramanian H, et al. Management of difficult-to-treat atopic dermatitis. J Allergy Clin Immunol Pract. 2013;1(2):142-151. doi: 10.1016/j.jaip.2012.09.002.
Kholodova IN. Osobennosti naruzhnoi terapii allergicheskikh zabolevanii kozhi u detei [Peculiarities of external therapy of allergic skin diseases in children]. Med Sovet. 2022;16(1):143-148. Russian. https://doi.org/10.21518/2079-701X-2022-16-1-143-148.
Kobzar' AI. Prikladnaia matematicheskaia statistika. Dlya inzhenerov i nauchnykh rabotnikov [Applied mathematical statistics. For engineers and scientists]. 2nd ed. Moscow: Fizmatlit; 2012. 816 p. Russian.

