Introduction
There is increasing evidence linking exposure to increased oxygen concentration and oxidative stress (OS) to the development of chronic bronchopulmonary disease, making the lung of preterm infants more susceptible to various diseases such as respiratory distress syndrome, bronchopulmonary dysplasia (BPD), and persistent pulmonary hypertension. Existing research in the field shows that although BPD is a disease with a multifactorial pathogenesis, the major risk factors are exposure to hyperoxia and the action of reactive oxygen species (ROS) [1, 2].
From conception to birth, mammals (including humans) develop under conditions of physiological hypoxia, with fetal arterial and venous pO2 values rarely exceeding ~4 kPa (30 mmHg) which constitutes ~4% O2. Thus, the entire process of organogenesis takes place in hypoxia, despite the fact that fetal hemoglobin has a significantly higher affinity for oxygen compared to that of an adult. The birth process is accompanied by an increase of oxidative stress, and this continues in the first months of life due to exposure to increased concentrations of oxygen provided by breathing. The role of oxidative stress triggered by hyperoxia in development is still unclear, but ROS are known to be involved in signal transduction, they also play an important role in immune function, are important regulators of circulation, activate cellular growth factors, remove dysfunctional proteins by oxidation and are essential for the functioning of cellular organelles [3].
It has been demonstrated that before and at birth in infants there is an increase in signs of oxidative stress, which indirectly reveals the increase in antioxidant capacity with the aim of adapting to the amplification of oxidative processes induced by the spontaneous inhalation of oxygen. At the same time, these adaptive reactions are missing or significantly underdeveloped in premature babies, especially those very premature and those with very low birth weight. Deficiencies of antioxidant protection mechanisms in these children are exacerbated by pulmonary insufficiency, lack of alveolar surfactant, underdevelopment of antioxidant enzymes. Subsequently, premature infants are much more likely to be hyperoxic and will develop oxidative stress following even short-term exposure to high levels of oxygen [4].
As a result of this phenomenon, cellular homeostasis is affected. The balance between the production and excessive accumulation of reactive oxygen species (ROS) in cells and the detoxification capacity due to the lack of endogenous antioxidants is inclined towards the amplification of oxidative processes, which causes tissue damage. Exogenous factors that stimulate ROS production have been shown to have both beneficial and deleterious cellular effects, thus either participating in cellular signaling or causing macromolecular damage [1, 2].
In case of the preterm babies, prolonged exposure to elevated oxygen concentrations can affect and alter the normal development of the lung tissue, triggering developmental disorders such as BPD. Some relevant studies regarding human BPD reported that increased ROS production is associated with impaired lung development [5].
Free radicals have unpaired electrons and are extremely reactive, but at the same time unstable, having low activation energy and short lifetime. It should be noted that they can act as both oxidants and antioxidants (reducers), a phenomenon explained by their ability to donate or accept an electron from other molecules [6].
The generation of ROS occurs in a series of redox reactions, which are the basis of many processes that take place in cells. Under physiological conditions, ROS are produced by the body as part of normal metabolic processes such as the electron transporting chain, Fenton and Haber-Weiss reactions etc. A series of pathological conditions and diseases, including those associated with hypoxia and ischemia/reperfusion, can disrupt the balance between the generation of ROS and the antioxidant system capacity to neutralize them, which causes oxidative stress. The amplification of the levels of intracellular ROS, causes damage of different macromolecules which will alter their function and the cell state [7, 8].
Oxidative stress is often associated with nitrosative stress, due to the interaction of nitric oxide with superoxide radical anion with the formation of peroxynitrite (ONOO‾), which, being a highly reactive radical, can cause enzyme inhibition, lipid peroxidation, protein and DNA damage, etc. The multitude of harmful processes initiated by NO metabolites can end with the induction of apoptosis and significant tissue damage [9-11].
Lipid hydroperoxides (LOOH), unsaturated aldehydes (MDA, 4-hydroxy-2-nonenal, 2-propenal or acrolein) and isoprostanes are relatively stable primary products of the lipid peroxidation process [6]. The well-known product of lipid damage produced by oxidative stress, malonic dialdehyde (MDA), is a recognized biomarker of oxidative stress, cell membrane damage, but also tissue and cell oxidative damage [12]. MDA is formed, as a result of the peroxidation process of polyunsaturated FA, either in the presence of a large number of free oxygen radicals from sialic acid and deoxyribose, or from the phospholipid structure of cell membranes [13, 14].
MDA is considered the most mutagenic product, in contrast to 4-hydroxy-2-nonenal, which is considered the most toxic. There are three mechanisms by which the damaging effect of lipid oxidation products is explained: the damage to the integrity of the cell membrane, the ability to add an additional ROS gene, or the degradation into reactive compounds, which have the potential of damaging DNA, proteins and lipids [6, 7]. The effects of lipid peroxidation in cells are loss of cell membrane properties, inactivation of many membrane receptors, and increased influx of calcium ions. These events alter permeability, membrane electrical potential and intercellular communication [15].
A number of commonly used methods for the assessment of oxidative/nitrosative stress (methods for measuring lipid and protein oxidation end products) are described. Nevertheless, there is an issue that is still addressed – the ability of oxidative/nitrosative stress, measured in plasma, to reflect the tissue processes, along with the need for a simple laboratory method to characterize an oxidative stress „profile” related to growth and maturation in physiological conditions and different diseases [15].
In children with DBP who endure chronic hypoxia due to respiratory impairment, we assume an alteration of these mechanisms. And our study was initiated as a challenge to have answers to these complex questions.
The aim of the research was to evaluate markers of oxidative/nitrosative stress measured in the serum of preterm children with bronchopulmonary dysplasia and to analyze their performance as a diagnostic test.
Material and methods
The research was carried out as part of the doctoral project „Prooxidant and antioxidant status in bronchopulmonary dysplasia in premature children”.
To describe the results of the assessment of oxidative stress markers in children with BPD, an analytical analysis based on a cohort study was performed. In this regard, 81 follow-up records of patients admitted to the Institute of Mother and Child (Chisinau, Republic of Moldova) with positive history of preterm births, postnatal oxygen therapy in respiratory distress were documented and analyzed. The patients were examined according to the same protocol, which included the complex examination and contained the information from the outpatient medical record (F112/e), the inpatient medical record (F003/e). Comparison groups were evaluated prospectively, through clinical, laboratory, instrumental examination. The children were divided into main group (children born preterm with BPD) and control group (children born preterm without BPD). Data analysis was performed according to the methodology described in „Basics of Epidemiology and Research Methods” [16].
The biochemical investigations were carried out according to methods adapted by the collaborators of the Biochemistry Laboratory of Nicolae Testemițanu State University of Medicine and Pharmacy for the Synergy H1 (Hydrid Reader) microplate spectrofluorometer (BioTek Instruments, USA) and the Power Wave HT spectrophotometer (BioTek Instruments, USA).
For the analysis of markers of interest, venous blood samples (5 mL) were collected, which were centrifuged for 10 minutes at 3000 revolutions/minute. The serum was separated and transferred to Eppendorf tubes and stored at -45°C separately until biochemical testing. All samples were coded.
The prooxidant-antioxidant balance (PAB) was performed by the method described by Toloue Pouya V. et al. [17], modified by Pantea V. et al. [18]. The method is based on the capacity of the free radicals, peroxides and antioxidants, contained in the blood sample, to interact with the TMB (3,3′,5,5′-tetramethylbenzidine) or TMB cation, that will determine changes of the solution color. PAB values were calculated according to the calibration curve data and expressed in arbitrary units.
Determination of oxidative stress marker – malonic dialdehyde and total prooxidant activity, was performed according to the procedure described by Galaktionova LP. et al. [19], and modified by Gudumac V. et al. [20]. The method is based on the spectrophotometric identification of the colored trimethine complex, resulting from the interaction of thiobarbituric acid with DAM. The concentration of DAM in the sample is directly proportional to the intensity of the staining. The final result was expressed in μM/L.
Determination of nitrosative stress marker – nitric oxide metabolites, was performed according to the procedure described by Меtеlskaya VА. et al. [21], modified by Gudumac V. et al. [22]. The principle of the method consists in the deproteinization of the biological material, the reduction of nitrates into nitrites, the processing of the supernatant with the Griss reagent, and the subsequent measurement of the optical density of the reaction product. The calculation of the nitrite concentration was carried out with the help of the calibration curve, built on the basis of successive dilutions of the standard solution of sodium nitrite and was expressed in μmol/L.
The data were statistically processed by operating electronic computerized assessment techniques of the degree of relationship between the evaluated parameters of the patients in the study groups, using Microsoft Excel, MedCalc (DeLong et al., 1988) and SPSS and Contingency Table Analysis as a way to summarize the performance of a diagnostic test [23-25].
Results
An oxidant is any compound that can accept electrons, including oxygen. On the other hand, a substance that donates electrons, is a reducing agent. The redox reactions are essential to the many processes that take place in cells. For specific biological systems, the terms prooxidant and antioxidant are equivalent in chemistry to the terms oxidant and reductant [15]. Many radicals are unstable and highly reactive. Behaving as oxidants or reductants, they have the ability to give an electron or accept an electron from other molecules, and homeostasis between them is important for optimal functioning of the system [14].
The evaluation of the redox status in the blood of the preterm children with and without BPD revealed the prevalence of oxidative processes in the children of the main group compared to the children of the control group (table 1).
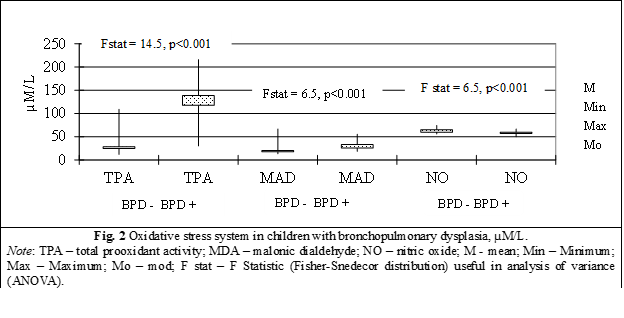
The values of total prooxidant activity (TPA) in children with BPD (41 children) was equal to 138.2±6.1 µM/L, value significantly increased compared to TPA in children without BPD (40 children) equal to 28.4±2.3 µM/L, Fstat = 14.5, p < 0.0001 (table 1, fig. 2).
A 4.86-fold increase (p < 0.001) of the total prooxidant activity was identified in preterm children with BPD compared to those without lung damage, a phenomenon that reveals the intensification of the production and accumulation of prooxidants of different nature, which can amplify the oxidation processes up to the level of oxidative/nitrosative stress (table 1, fig. 2).
Table 1. Values of markers of oxidative stress in children with bronchopulmonary dysplasia | |||||
Marker | Control group (n = 40) | Main group (n = 41) | The veracity of differences between groups | ||
TPA (μM/L) | 28.4±14.3 | 100% | 138.2±38.9 | 486% | p < 0.001 |
PAB (U) | 140.3±15.2 | 100% | 99.6±15.8 | 71% | p < 0.001 |
MDA (μM/L) | 20.4±8.2 | 100% | 33.0±8.9 | 162% | p < 0.001 |
NO metabolites (μM/L) | 64.9±3.8 | 100% | 57.1±4.8 | 88% | p < 0.001 |
Note: TPA – total prooxidant activity; PAB – prooxidant/antioxidant balance; MDA – malonic dialdehyde; NO – nitric oxide. Variables are presented as Mean±SD. | |||||
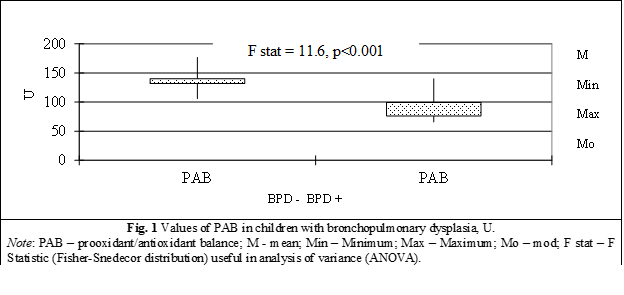
PAB in children with BPD (41 children) is equal to 99.6±2.5 µM/L with minimum value of 65.8 µM/L, median – 101.8 µM/L, maximum – 140.3 µM/L, mode – 76.4 µM/L, compared to PAB concentration in children without BPD (40 children) which have a significant difference between groups equal to 140.3±2.4 µM/L (minimum value of 105.9 µM/L, median – 140.7 µM/L, maximum – 176.8 µM/L, mode – 131.7 µM/L), Fstat = 11.6, p < 0.00001 (table 1, fig. 2).
A major, statistically significant decrease of PAB by 29% (p < 0.001) was identified in preterm children from the group with bronchopulmonary dysplasia compared to children from the control group, which attests to the decrease in the total level of antioxidants with the inclination of the balance towards the formation and accumulation of prooxidants and the definite establishment of the prooxidant status (fig. 1).
Malonic dialdehyde (MDA), in children with BPD (41 children) was 33.1±1.39 µM/L with minimum value of 18.3 µM/L, median – 31.8 µM/L, maximum – 55.4 µM/L, mode – 25.4 µM/L, compared to the MDA level in children without DBP (40 children) which was 20.4±1.3 µM/L (minimum value of 12.9 µM/L, median – 19.1 µM/L, maximum – 66.8 µM/L, mode – 17.3 µM/L), and presents a significant difference between batches (Fstat = 6.5, p < 0.0001) (table 1, fig. 2).
The installation of the prooxidant status in preterm children from the group with bronchopulmonary dysplasia, initiated the atypical oxidation of lipids via the peroxidative pathway, which was manifested by a significant increase of 62% (p < 0.001) in the content of MDA, the final product of the peroxidation of unsaturated fatty acids, mainly from cell membranes phospholipids (fig. 2).
Nitric oxide (NO) in children with BPD (41 children) is equal to 57.13±0.75 µM/L with minimum value of 49.6 µM/L, median – 57.4 µM/L, maximum – 66.7 µM/L, mode – 59.7 µM/L, compared to the concentration of nitric oxide in children without BPD (40 children) which has a significant difference between groups equal to 64.9±0.6 µM/L (minimum values of 55.8 µM/L, median – 65.1 µM/L, maximum – 74.4 µM/L, mode – 59.7 µM/L), Fstat = 7.9, p < 0.00001 (table 1, fig. 2).
A statistically significant decrease in the level of NO metabolites by 12% (p < 0.001) was revealed in premature infants from the group with bronchopulmonary dysplasia compared with children in the control group, which may indicate the use of NO in reactions that cause reactive forms of nitrogen (peroxynitrite, protonated peroxynitrite), thereby contributing to the induction of nitrosative stress and the deepening of the redox imbalance (fig. 2).
We can conclude that preterm children with BPD are characterized by the amplification of ROS/NRS production reactions, the establishment of a prooxidant status and the triggering of OS/NS, which ultimately causes damage to biomolecules and cellular macromolecular structures (membranes).
Next are presented the derivations of multiple measures using the four outcomes of the 2×2 contingency table for the prooxidant system to assess utility as diagnostic tests in children with BPD.
The analysis using ROC curves (Receiver Operating Characteristics) was chosen as a statistical model. These are two-dimensional curves of the values of a diagnostic test that ends with the evaluation of this examination applied to each patient or their comparison [23].
The assessment of the usefulness of determining the TPA, for highlighting children at risk of developing oxidative stress, was carried out by means of the ROC curve, which is an excellent way to compare diagnostic tests (fig. 3).
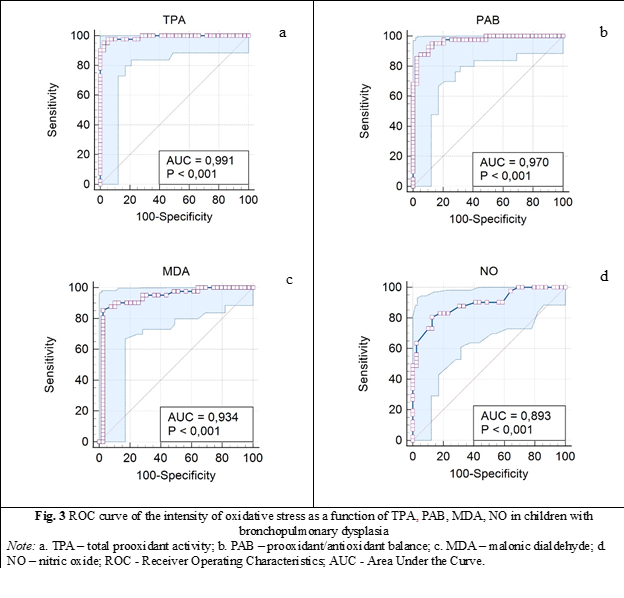
When the variable under study cannot distinguish between the two groups, i.e., where there is no difference between the two distributions, the area will be equal to 0.5 (the ROC curve will coincide with the diagonal). When there is a perfect separation of the values of the two groups, i.e., there is no overlapping of the distributions, the area under the ROC curve equals 1 (the ROC curve will reach the upper left corner of the plot) (fig. 3). A reflection of the identification of prooxidant processes depending on TPA can be the area located below the level of the ROC curve equal to 0.99 (AUC is equal to 0.991: 95%CI 0.98-1, p = 0.000) (table 2). So, our study confirms the importance of evaluating prooxidant process according to TPA concentration, which has been shown to be a useful test.
Area under the curve of the ROC curve for PAB values was obtained equal to 0.97: 95% CI 0.905-0.999, p = 0.000 (table 2).
Table 2. Area under the curve (AUC) for the values of markers of oxidative stress in children with bronchopulmonary dysplasia. | |||||
| AUC | Standard Error | p | 95% Confidence Interval | |
Lower Bound | Upper Bound | ||||
TPA (μM/L) | 0.991 | 0.008 | 0.000 | 0.976 | 1.000 |
PAB (U) | 0.97 | 0.016 | 0.000 | 0.905 | 0.995 |
MDA (μM/L) | 0.934 | 0.032 | 0.000 | 0.871 | 0.997 |
NO (μM/L) | 0.893 | 0.035 | 0.000 | 0.804 | 0.951 |
Note: TPA – total prooxidant activity; PAB – prooxidant/antioxidant balance; MDA – malonic dialdehyde; NO – nitric oxide; AUC - Area Under the Curve; p – signification. | |||||
In the case of the malondialdehyde test, the minimum sensitivity of the test was of 2.4%: 95%CI, 0.01-0.12 for MDA concentration less than 20 µM/L (characteristic only of a child with DBP). The specificity was also minimal with the highest value reaching only 4.9%: 95%CI, 0.05-0.6, which confirms levels higher than 20 µM/L of MDA in only 20 children from the control group (without DBP), χ2 = 24.6, p < 0.0001 (fig. 3).
Area under the ROC curve equal to 0.934: 95%CI 0.87-0.99, p = 0.000 values for MDA content (µM/L) in children with BPD (fig. 3, table 2).
And the last demonstration concerns the area under the curve of NO – nitric oxide which was obtained equal to 0.893: 95%CI 0.804-0.951, p = 0.000 (table 2).
Discussions
According to literature data, multiple harmful gestational factors, which can affect the growth and development of the product of conception during the entire intrauterine period, are reported.
During postnatal development, the preterm born child is even more influenced by the convergence of the multitude of endogenous and exogenous factors that have damaged the health status. A dominant focus of the modern experimental studies demonstrates the harmfulness of oxidative stress, as the ultimate goal through the generation of free radicals (FR) and as a result the occurrence of cellular, tissue and organic damage [26, 27].
Our study, based on a prospective evaluation of two groups of children: the main group - preterm infants with BPD and the control group - preterm infants who did not develop BPD, examined clinically, paraclinical and instrumentally, contributes to the study of changes in oxidative stress markers in preterm infants who have developed bronchopulmonary dysplasia, the analysis of these data using various contingency tables and their generalization by performing a diagnostic test.
In multiple series of papers, and a huge variety of laboratory methods and statistical models have been developed and used to measure oxidative stress intensity and its consequences. Researchers such as Ferguson K, Gunko V O, Abiaka C, Machado L in their studies, determine biomarkers using various methods [28-30]. At the same time, all these methods are considered to be quite difficult, since oxidative stress biomarkers are very reactive and have very short half-lives.
Thus, the conducted study is within the limits of the research level, which is of scientific value in terms of the assessed markers of oxidative stress. These markers evaluated in premature children with bronchopulmonary dysplasia compared to those without this lung damage revealed the presence of significant oxidative stress in those enrolled in the main group. Total prooxidant activity increased by 386% (p < 0.001), the balance between prooxidants and antioxidants shifted towards the former (PAB - 29%, p < 0.001) and at the same time the NO content significantly decreased (-12%, p < 0.001). Our research also found lipid damage with the accumulation of the end product of lipid peroxidation – DAM (+62%, p < 0.001).
However, the role of oxidative stress in neonatal lung injury is much more complex and not fully studied. Despite the multiple existing data and different research environments under development, clinical practice is limited in the ability to detect very early preterm newborns who have susceptibility to develop a lung pathology, therefore the markers used currently cannot fully predict the lung damage that may follow in these children. But the detection and monitoring of lung lesions related to oxidative stress, namely through the use of non-invasive methods of detecting different oxidation products, remains to have a predictive and very useful role in the clinical setting.
While a growing body of evidence supports the role of oxidative stress, it appears that the complexity of this multifactorial condition cannot be captured by a single marker. Instead, researchers should move on to develop and validate specific panels of biomarkers that can more reliably predict certain pathological states that evolve with impaired lung function. Early diagnosis and treatment of oxidative stress-related lung diseases may be essential to prevent adverse effects that may spread beyond the neonatal period. Indeed, few of the biomarkers developed to date have been qualified for neonatal lung disease and their analysis has been limited by research settings.
Our study confirms that TPA, PAB, MDA and NO values are reliable markers of hypoxic tissue damage in children with bronchopulmonary dysplasia and can be recommended for assessing the intensity of oxidative stress. Last but not least, the currently available evidence, including our results highlights the need for further studies on a larger scale and with longer follow-up periods to obtain more precise results and allow serial detection of oxidative stress biomarkers.
Conclusions
Oxidative stress is a major contributor to lung injury in preterm children with bronchopulmonary dysplasia, fact confirmed by significantly higher values of total prooxidant activity (4.86 times, p < 0.001) and MDA (by 62%, p < 0.001) along with concomitant decrease of the prooxidnat/antioxidant and NO metabolites levels in the blood of these children. The phenomenon reveals an increase in the production and accumulation of prooxidants of various nature, which enhances oxidation processes and causes damage to biomolecules and cellular macromolecular structures, membranes in particular.
Thus, our study confirms the importance of evaluating pro-oxidant processes according to the concentrations of TPA, PAB, MDA, NO, which have been demonstrated as useful tests.
Competing interests
None declared.
Authors’ contribution
Authors contributed equally to the literature searching, conceptual highlighting of the material as well as writing of the manuscript. The authors read and approved the final version of the manuscript.
Authors’ ORCID IDs
Mariana Ceahlau – https://orcid.org/0009-0009-3322-344X
Rodica Selevestru – https://orcid.org/0000-0002-8923-3075
Olga Tagadiuc – https://orcid.org/0000-0002-5503-8052
Svetlana Șciuca – https://orcid.org/0000-0003-1091-9419
References
Maltepe E, Saugstad O. Oxygen in health and disease: regulation of oxygen homeostasis - clinical implications. Pediatr Res. 2009;65(3):261-268. https://doi.org/10.1203/PDR.0b013e31818fc83f.
Kaarteenaho-Wiik R, Kinnula VL. Distribution of antioxidant enzymes in developing human lung, respiratory distress syndrome, and bronchopulmonary dysplasia. J Histochem Cytochem. 2004;52(9):1231-1240. doi: 10.1369/jhc.4A6291.2004.
Wang J, Dong W. Oxidative stress and bronchopulmonary dysplasia. Gene. 2018;678:177-183. doi: 10.1016/j.gene.2018.08.031.
Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Oxford: Clarendon Press, Oxford University Press; 2000. p. 160-5.
Chandrasekaran A, Idelchik MDPS, Melendez JA. Redox control of senescence and age-related disease. Redox Biol. 2017;11:91-102. doi: 10.1016/j.redox.2016.11.005.
Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signaling agents. Nat Rev Mol Cell Biol. 2020 Jul;21(7):363-383. doi: 10.1038/s41580-020-0230-3.
Liguori I, Russo G, Curcio F, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;2018(13):757-772. doi: 10.2147/CIA.S158513.
Pavlovschi E. Markerii biochimici ai retinopatiei hypertensive [Biochemical markers of hypertensive retinopathy] [dissertation]. Chișinău: Doctoral School in Medical Sciences; 2022. 118 p. Romanian.
Lazăr C, Vozian M, Pantea V, Şveţ I, Mishina A, Tagadiuc O. The effect of controlled reperfusion on experimental ovarian torsion. Russ Open Med J. 2019;8(4):1-5. doi: 10.15275/rusomj.2019.0404.
Riggins JN, Marnett LJ. Mutagenicity of the malondialdehyde oligomerization products 2-(3'-oxo-1'-propenyl)-malondialdehyde and 2,4-dihydroxymethylene-3-(2,2-dimethoxyethyl) glutaraldehyde in Salmonella. Mutat Res. 2001 Oct 18;497(1-2):153-7. doi: 10.1016/s1383-5718(01)00253-4.
Moris D, Spartalis M, Tzatzaki E, Spartalis E, Karachaliou GS, Triantafyllis AS, Karaolanis GI, Tsilimigras DI, Theocharis S. The role of reactive oxygen species in myocardial redox signaling and regulation. Ann Transl Med. 2017 Aug;5(16):324. doi: 10.21037/atm.2017.06.17.
Clark RH, Gerstmann DR, Jobe AH, Moffitt ST, Slutsky AS, Yoder BA. Lung injury in neonates: causes, strategies for prevention, and long-term consequences. J Pediatr. 2001 Oct;139(4):478-86. doi: 10.1067/mpd.2001.118201.
Granot E, Kohen R. Oxidative stress in childhood - in health and disease states. Clin Nutr. 2004 Feb;23(1):3-11. doi: 10.1016/s0261-5614(03)00097-9.
Spinei L, Stefăneţ S, Moraru C. Notiuni de baza de epidemiologie şi metode de cercetare [Basic knowledge of epidemiology and research methods]. Chisinau: Bons Offices; 2006. 224 p. Romanian.
Toloue Pouya V, Hashemy SI, Shoeibi A, Nosrati Tirkani A, Tavallaie S, et al. Serum prooxidant-antioxidant balance, advanced oxidized protein products (AOPP) and protein carbonyl in patients with stroke. Razavi Int J Med. 2016;4(2):e38203.
Gudumac V, Tagadiuc O, Rîvneac V, Sardari V, Pantea V, et al. Investigații biochimice. Vol. 2: Micrometode [Biochemical investigations. Vol. 2: Micromethods]. Chisinau: “Elena-VI”; 2010. 97 p. ISBN 978-9975-106-06-1. Romanian.
Băicuş C. Medicina bazată pe dovezi: cum înţelegem studiile [Evidence-based medicine: how we understand studies]. Bucharest: Editura Medicală; 2007. 144 p. Romanian.
Everitt BS. The Cambridge dictionary of statistics. 2nd ed. Cambridge, UK: Cambridge University Press; 2002. 410 p. ISBN 052181099X.
Powers, DMW. Evaluation: from precision, recall and F-score to ROC, informedness, markedness & correlation. J Mach Learning Technol. 2011;2(1):37-63.
Ferrante G, Carota G, Li Volti G, Giuffrè M. Biomarkers of oxidative stress for neonatal lung disease. Front Pediatr. 2021 Feb 18;9:618867. doi: 10.3389/fped.2021.618867.
Perrone S, Santacroce A, Picardi A, Buonocore G. Fetal programming and early identification of newborns at high risk of free radical-mediated diseases. World J Clin Pediatr. 2016;5(2):172-81. doi: 10.5409/wjcp.v5.i2.172.
Arogbokun O, Rosen E, Keil AP, Milne GL, Barrett E, Nguyen R, Bush NR, Swan SH, Sathyanarayana S, Ferguson KK. Maternal oxidative stress biomarkers in pregnancy and child growth from birth to age 6. J Clin Endocrinol Metab. 2021 Apr 23;106(5):1427-1436. doi: 10.1210/clinem/dgab018.
Abiaka C, Machado L. Nitric oxide and antioxidant enzymes in venous and cord blood of late preterm and term omani mothers. Sultan Qaboos Univ Med J. 2012 Aug;12(3):300-5. doi: 10.12816/0003143.
Gunko VO, Pogorelova TN, Linde VA. Proteomic profiling of the blood serum for prediction of premature delivery. Bull Exp Biol Med. 2016;161(6):829-832. https://doi.org/10.1007/s10517-016-3522-z.

