Introduction
Currently, there is a focus on psychophysiological research in the field of breathing, aiming to understand how various controlled respiratory patterns influence heart rate variability (HRV) [1]. Abdominal (diaphragmatic) breathing, an essential component of protocols that enhance the amplitude of Respiratory Sinus Arrhythmia (RSA), forms the basis of treatment methods for a range of stress-related conditions and autonomic dysfunctions [2, 3].
Respiratory Sinus Arrhythmia (RSA) is characterized by rhythmic fluctuations in heart rate (HR) throughout the respiratory cycle. HR increases during inspiration and decreases during expiration. RSA, as a component of Heart Rate Variability (HRV), is regarded as an indicator of autonomic homeostasis and adaptability [4]. However, despite numerous studies on this subject, much remains unknown regarding the relationship between specific respiratory strategies and RSA [5].
HRV measurements encompass both time and frequency domain variables. Frequency domain HRV metrics include low frequency power (LF), high frequency power (HF), normalized low frequency power (LFn), normalized high frequency power (HFn), and the LF/HF ratio. In healthy adults, the typical resting breathing rate ranges from 9 to 24 breaths per minute [3]. Respiratory sinus arrhythmia (RSA), which is modulated by the parasympathetic nervous system (PNS), occurs within the high-frequency range of 0.15-0.4 Hz [6, 7, 8]. LF serves as a marker of the cardiac sympathetic nervous system (SNS) [8, 9]; however, some studies have not been able to confirm this association [10, 11]. Several studies have suggested that LF is likely influenced by both the SNS and PNS, as well as baroreflex modulation of autonomic flows [11-14].
Previously, the LF/HF ratio was considered an indicator of cardiac autonomic balance, where an increase in the ratio indicated SNS dominance, and a decrease indicated PNS dominance [8]. However, recent studies have demonstrated that the LF/HF ratio may not necessarily reflect SNS or PNS influence [6, 13, 15]. The LF/HF ratio is influenced by various factors, including vagal activity, SNS activity, and respiratory parameters [13, 14], and its interpretation should take into account the individual variations of LF and HF components of heart rate variability [13].
The objective of the study was to identify predictors associated with the modulation of sympathovagal balance, as expressed by the LF/HF index, utilizing respiratory pattern parameters recorded during abdominal breathing.
Materials and methods
The study was conducted on a group of 101 subjects from March 2017 to February 2019 at the Department of Human Physiology and Biophysics, Nicolae Testemiţanu State University of Medicine and Pharmacy. The average age of the individuals included in the study was 33.5 years (ranging from 19 to 60 years old). Subjects with pulmonary and cardiac pathologies were excluded.
All participants signed an informed agreement to be included in this study, which was approved by the Ethical Committee of “Nicolae Testemițanu” State University of Medicine and Pharmacy, with minutes no. 15 dated 11.01.2016.
The recording of breathing patterns was performed using a respiratory induction plethysmograph (RIP) VISURESP (RBI instruments, France) to measure movements of the abdomen and thorax [16]. Additionally, the capnograph CapnoStreamTM 20 (Medtronic, USA) was used to record the partial pressure of CO2 in the expired air at the end of expiration (etCO2). The respiratory parameters measured included tidal volumes (Vt), the duration of the respiratory cycle (Tt), respiratory frequency (FR), inspiratory time (Ti), expiratory time (Te), average inspiratory flow (Vt/Ti), respiratory minute volume (MVR), and etCO2. The recording of ECG signals was performed using the computer system Biopac MP-100. The data processing was conducted using the software Kubios HRV Standard (version 3.2.0, 2019), with manual removal of artifacts. The spectral analysis of the RR interval variation involved calculating the power of the components: LF (low frequency power, in ms²) in the 0.04-0.15 Hz range, and HF (high frequency power, in ms²) in the > 0.15 Hz range.
The experimental protocol included recording the respiratory signals and ECG in a supine position. During the recording, the subjects were asked to breathe quietly, not talk, and avoid additional movements.
- Resting period (RR) - for 5 minutes in physical, mental, and emotional rest periods (the first minute was excluded from calculations to exclude artifacts obtained from the application and accommodation movements of subjects in the device's jacket).
- Abdominal respiration (AR) - the subjects used abdominal (diaphragmatic) breathing. To perform this type of breathing, the movement of the rib cage was restricted using a chest corset.
The statistical analysis included descriptive statistics, multivariate statistics (ANOVA), and regression analysis. The analysis was performed using IBM SPSS Statistics version 22.0 software (Statistical Package for the Social Sciences 22.0, IBM Corp., Armonk, NY, USA).
Results
Our study utilized seven parameters of the breathing pattern as presumed predictors of the LF/HF ratio. These parameters were recorded during resting breathing and abdominal breathing in the subjects. We developed predictive models for each type of breathing, incorporating these parameters.
Resting respiration. The descriptive analysis of the research group, subjected to statistical analysis (Table 1), revealed the following findings:
- Tidal volume: The tidal volume ranged from 0.27 l to 0.66 l, with an average of 0.466 l. The standard deviation was 0.1012;
- Inspiratory time at rest: The inspiratory time varied between 1.15 s and 2.41 s, with an average of approximately 1.64 s. The standard deviation was 0.3555;
- Duration of free expiration: The duration of free expiration ranged from 1.14 s to 4.64 s. The average duration was 4.64 s, with a standard deviation of 0.8714;
- Total duration of respiratory cycle: The total duration of the respiratory cycle ranged from 2.32 s to 7.05 s. The mean duration was 4.06 s, with a standard deviation of 1.17 s;
- Vt/Ti ratio: The Vt/Ti ratio varied between 0.20 l/s and 0.39 l/s. The mean value was 0.287, with a standard deviation of 0.055;
- Respiratory volume per minute: At rest, the respiratory volume per minute oscillated between 4.49 l and 10.16 l, with a respiratory rate ranging from 8.50 c/min to 24.53 c/min. The average minute respiratory volume was 7,094 l/min, with a standard deviation of 1,591 l;
- Respiratory rate: The average respiratory rate at rest was 15.9 c/min, with a standard deviation of 4.2;
LF/HF index: At rest, the LF/HF index ranged from 0.18 to 5.80. However, the average LF/HF index was 1.066, with a standard deviation of 1.4459.
Table 1. Descriptive statistics of researched group in resting period. | |||||
| N | Minimum | Maximum | Mean | Std. deviation |
Vt | 15 | .27 | .66 | .4667 | .10123 |
Ti | 15 | 1.15 | 2.41 | 1.6383 | .35553 |
Te | 15 | 1.14 | 4.64 | 2.4211 | .87141 |
Tt | 15 | 2.32 | 7.05 | 4.0593 | 1.17248 |
Vt/Ti | 15 | .20 | .39 | .2872 | .05579 |
MVR | 15 | 4.49 | 10.16 | 7.0935 | 1.59086 |
FR | 15 | 8.50 | 24.53 | 15.9162 | 4.20697 |
CC | 15 | .70 | 1.15 | .8827 | .14144 |
LF/HF | 15 | .18 | 5.80 | 1.0662 | 1.44592 |
Note: Vt – tidal volume; Ti – duration of inspiration; Te – duration of expiration; Tt – duration of respiratory cycle; Ti/Tt – ratio of inspiration in respiratory cycle; Vt/Ti – inspiratory flow; MVR – respiratory minute volume; FR – breathing rate; CC – duration of cardiac cycle; LF/HF – ratio of low frequency power to high frequency power of HRV. | |||||
The possible complex interactions between the measured factors argued for the need for multivariate analysis. Consequently, a model (RR model) was developed with the objective of predicting the balance between sympathetic and parasympathetic activity based on the LF/HF ratio. The model incorporated the standardized values of tidal volume, total respiratory cycle time, respiratory frequency, and minute respiratory volume as predictors (Table 2).
Table 2. Model summary for RR model. | ||||
Model | R | R squared | Adjusted R squared | Std. error of the estimate |
| .880 | .75 | .684 | .56170691 |
Predictors: (Constant), Zscore (Tt), Zscore (Vt), Zscore (FR), Zscore (MVR) | ||||
Dependent variable: Zscore (LF/HF) | ||||
Note: Zscore (LF/HF) – standardized score of the ratio of low frequency power to high frequency power of HRV; Zscore (Tt) – standardized score of the duration of respiratory cycle; Zscore (Vt) – standardized score of the tidal volume; Zscore (FR) – standardized score of the breathing rate; Zscore (MVR) – standardized score of respiratory minute volume. | ||||
The multivariate analysis conducted on the resting values was able to explain 68.4% of the changes in LF/HF balance. The coefficient of determination (Adjusted R Square) was 0.684, indicating that the proposed model accounted for a significant portion of the variance in the LF/HF variable for resting breathing. The sum of squares was 10,845 out of a possible 14,000, further supporting the model's ability to explain more than two-thirds of the variance. The null hypothesis, which states that no parameter included in the model can predict the LF/HF value for resting breaths better than an arbitrary model, was rejected. This rejection was based on the statistical test result (F = 8.593, p = 0.003) as shown in Table 3.
Table 3. ANOVA test in RR model. | |||||
Model | Sum of squares | df | Mean square | F | Sig. |
Regression | 10.845 | 4 | 2.711 | 8.593 | .003 |
Residual | 3.155 | 10 | .316 |
|
|
Total | 14.000 | 14 |
|
|
|
Dependent variable: Zscore (LF/HF) | |||||
Predictors: (Constant), Zscore (Tt), Zscore (Vt), Zscore (FR), Zscore (MVR) | |||||
Note: df – degrees of freedom; F – Fisher’s coefficient; Zscore (LF/HF) – standardized score of the ratio of low frequency power to high frequency power of HRV; Zscore (Tt) – standardized score of the duration of respiratory cycle; Zscore (Vt) – standardized score of the tidal volume; Zscore (FR) – standardized score of the breathing rate; Zscore (MVR) – standardized score of the respiratory minute volume. | |||||
When developing the model, the Backward method was used. Initially, all potential variables were included in the model, and then insignificant parameters were systematically excluded until only the optimal combination of variables remained to form the regression equation and predict the studied outcome. The resulting model, presented in Table 4, included the constant (B = 3.310E-15, p = 1.000) and the standardized values of MVR (B = 1.731, p = 0.040), FR (B = 1.379, p = 0.049), Vt (B = -1.622, p = 0.062), and Tt (B = 3.580, p < 0.001). The final model requires attention and possible improvements because it did not include the constant, which is very close to 0. Additionally, the standardized value of Vt was found to be insignificant in this case, as its confidence interval included the value of 0. Therefore, further refinement of the model is necessary.
Based on the model, it was determined that the resting LF/HF value can be predicted using the following equation: LF/HF in resting breathing = Zscore (MVR) × 1.731 + Zscore (FR) × 1.379 – Zscore (Vt) × 1.622 + Zscore (Tt) × 3.580.
Table 4. Coefficients of predictors in RR model. | |||||||
Model | Unstandardized coefficients | Standardized coefficients | t | Sig. | 95.0% confidence interval for B | ||
B | Std. error | Beta | Lower bound | Upper bound | |||
(Constant) | 3.310E-15 | .145 |
| .000 | 1.000 | -.323 | .323 |
Zscore (MVR) | 1.731 | .733 | 1.731 | 2.363 | .040 | .099 | 3.363 |
Zscore (FR) | 1.379 | .614 | 1.379 | 2.246 | .049 | .011 | 2.747 |
Zscore (Vt) | -1.622 | .771 | -1.622 | -2.105 | .062 | -3.340 | .095 |
Zscore (Tt) | 3.580 | .703 | 3.580 | 5.090 | .000 | 2.012 | 5.147 |
Dependent variable: Zscore (LF/HF) | |||||||
Note: Zscore (Tt) – standardized score of the duration of respiratory cycle; Zscore (Vt) – standardized score of the tidal volume; Zscore (FR) – standardized score of the breathing rate; Zscore (MVR) – standardized score of the respiratory minute volume; Zscore (LF/HF) – standardized score of the ratio of low frequency power to high frequency power of HRV. | |||||||
The necessary conditions for linear regression residuals were met by the developed model. The analysis demonstrated an almost normal distribution of residuals and a lack of associations between predictive standardized values and standardized residuals (Fig. 1). Taken together, these findings allow us to consider the model suitable.
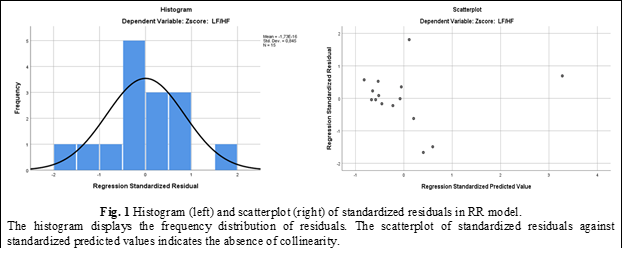
Abdominal respiration. The effects of physiological parameters recorded during the functional abdominal breathing test were considered as predictors of the LF/HF ratio. To investigate these relationships, an additional model was developed, incorporating the values obtained during resting breathing as well as the newly recorded parameters during abdominal breathing.
The current volume observed in individuals practicing abdominal breathing ranged from 0.37 to 0.64 liters, with an average of 0.496 liters and a standard deviation of 0.085. The duration of inspiration varied from 1.16 to 2.28 seconds, with a mean of 1.68 seconds and a standard deviation of 0.337 seconds. Expiration, on the other hand, had a longer duration than inspiration, ranging from 1.67 to 3.27 seconds. The mean duration of expiration was 2.58 seconds with a standard deviation of 0.43 seconds. The total time of a respiratory cycle ranged from 3.09 to 5.31 seconds, with an average of 4.26 seconds and a standard deviation of 0.67 seconds.
The minute ventilation rate (MVR) measured in the study participants varied between 5.1 and 9.93 liters per minute, with an average of 7.1 liters per minute and a standard deviation of 1.4 liters per minute. Respiratory frequency among patients practicing abdominal breathing ranged from 11.3 to 19.4 breaths per minute, with an average of 14.42 breaths per minute and a standard deviation of 2.34 breaths per minute.
The dependent variable in the current study exhibited an equal ratio ranging from 0.11 to 1.13, with a mean of 0.41 and a standard deviation of 0.24.
Table 5. Descriptive statistics of researched group in resting period and abdominal breathing. | |||||
| N | Minimum | Maximum | Mean | Std. deviation |
VtB | 15 | .27 | .66 | .4667 | .10123 |
TiB | 15 | 1.15 | 2.41 | 1.6383 | .35553 |
TeB | 15 | 1.14 | 4.64 | 2.4211 | .87141 |
TtB | 15 | 2.32 | 7.05 | 4.0593 | 1.17248 |
MVRB | 15 | 4.49 | 10.16 | 7.0935 | 1.59086 |
FRB | 15 | 8.50 | 24.53 | 15.9162 | 4.20697 |
CCB | 15 | .70 | 1.15 | .8827 | .14144 |
LF/HFB | 15 | .18 | 5.80 | 1.0662 | 1.44592 |
Vt | 15 | .37 | .64 | .4959 | .08540 |
Ti | 15 | 1.16 | 2.28 | 1.6800 | .33696 |
Te | 15 | 1.67 | 3.27 | 2.5829 | .43274 |
Tt | 15 | 3.09 | 5.31 | 4.2622 | .67374 |
MVR | 15 | 5.10 | 9.93 | 7.1024 | 1.47426 |
FR | 15 | 11.30 | 19.40 | 14.4200 | 2.33703 |
CC | 15 | .72 | 1.10 | .8640 | .11783 |
LF/HF | 15 | .11 | 1.13 | .4184 | .24757 |
Note: VtB – tidal volume; TiB – duration of inspiration; TeB – duration of expiration; TtB – duration of the respiratory cycle; MVRB – respiratory minute volume; FRB – breathing rate; CCB – duration of the cardiac cycle; LF/HFB – ratio of low frequency power to high frequency, all recorded in breathing at rest. Vt – tidal volume; Ti – duration of inspiration; Te – duration of expiration; Tt – duration of respiratory cycle; MVR – respiratory minute volume; FR – breathing rate; CC – duration of cardiac cycle; LF/HF – ratio of low frequency power to high frequency, all recorded in abdominal respiration. | |||||
The current predictive model aimed to investigate the impact of the measured parameters on the balance between sympathetic and parasympathetic activity, as assessed by the LF/HF ratio, in individuals practicing abdominal breathing. This investigation was conducted using multivariate analysis. The predictive potential of standardized scores for tidal volume, inspiratory and expiratory time, total duration of the respiratory cycle, minute respiratory volume, respiratory rate, and heart rate was evaluated. These measurements were taken at rest and during abdominal breathing (Table 6).
Table 6. Model summary for AR model. | ||||
Model | R | R squared | Adjusted R squared | Std. error of the estimate |
| 0.928 | 0.861 | 0.610 | 0.62418749 |
Predictors: (Constant), Zscore (CC), Zscore (LF/HFB), Zscore (MVRB), Zscore (Te), Zscore (Ti), Zscore (TeB), Zscore (VtB), Zscore (FR), Zscore (Vt) | ||||
Dependent variable: Zscore (LF/HF) | ||||
Note: Zscore (CC) – standardized score of the duration of cardiac cycle; Zscore (LF/HFB) – standardized score of the ratio of low frequency power to high frequency; Zscore (MVRB) – standardized score of the respiratory minute volume; Zscore (Te) – standardized score of the duration of expiration; Zscore (Ti) – standardized score of the duration of inspiration; Zscore (TeB) – standardized score of the duration of expiration; Zscore (VtB) – standardized score of the tidal volume; Zscore (FR) – standardized score of the breathing rate; Zscore (Vt) – standardized score of the tidal volume. | ||||
The coefficient of determination (Adjusted R-squared) is 0.61, indicating that the developed model explains more than three-fourths of the variance in the variable of interest, which is the balance between sympathetic and parasympathetic activity assessed based on the LF/HF ratio in abdominal breathers. The sum of squares was 12.052 out of a possible 14. The null hypothesis, which states that none of the parameters included in the model can predict the balance between sympathetic and parasympathetic activity assessed based on the LF/HF ratio in people with abdominal breathing, was not rejected (F = 3.437, p = 0.094). The Fisher test was statistically insignificant.
Table 7. ANOVA test in AR model. | |||||
Model | Sum of squares | df | Mean square | F | Sig. |
Regression | 12.052 | 9 | 1.339 | 3.437 | .094 |
Residual | 1.948 | 5 | .390 |
|
|
Total | 14.000 | 14 |
|
|
|
Dependent variable: Zscore (LF/HF) | |||||
Predictors: (Constant), Zscore (CC), Zscore (LF/HFB), Zscore (MVRB), Zscore (Te), Zscore (Ti), Zscore (TeB), Zscore (VtB), Zscore (FR), Zscore (Vt) | |||||
Note: df – degrees of freedom; F – Fisher’s coefficient; Zscore (LF/HF) – standardized score of the ratio of low frequency power to high frequency power of HRV; Zscore (CC) – standardized score of the duration of cardiac cycle; Zscore (MVRB) – standardized score of the respiratory minute volume; Zscore (Te) – standardized score of the duration of expiration; Zscore (Ti) – standardized score of the duration of inspiration; Zscore (TeB) – standardized score of the duration of expiration; Zscore (VtB) – standardized score of the tidal volume; Zscore (FR) – standardized score of the breathing rate; Zscore (Vt) – standardized score of the tidal volume. | |||||
The coefficient of determination was significantly reduced after adjusting for the larger number of independent variables included in the prediction model for assessing the balance of sympathetic and parasympathetic activity based on the LF/HF ratio in subjects using abdominal respiration. In order to avoid including ineffective and unnecessary variables in the calculation model, the Backward method was also employed. Consequently, the coefficients presented in Table 8 were obtained.
As shown, the regression model was optimized by including constant values and standardized scores of Vt, Te, MVR, and LF/HF recorded during restful breathing, as well as standardized values of Vt, Ti, Te, FR, and CC recorded during abdominal breathing. Among all the variables included, the final multiple regression model for this specific scenario was represented by the equation:
LF/HF in people with abdominal breathing = Zscore (VtB) × 5.007 - Zscore (TeB) × 3.831 - Zscore (MVRB) × 4.415 + Zscore (LF/HFB) × 1.428 - Zscore (Vt) × 0.728 - Zscore (Ti) × 4.037 - Zscore (Te) × 4.194 - Zscore (FR) × 5.953 - Zscore (Vt) × 0.705.
In this final model, there are variables whose predictive power raises doubts due to statistical insignificance and the inclusion of the value 0 within the 95% confidence interval. However, their predictive value can be further explored in future research involving larger numbers of participants.
Table 8. Coefficients of predictors in AR model. | |||||||
Model | Unstandardized coefficients | Standardized coefficients | t | Sig. | 95.0% confidence interval for B | ||
B | Std. error | Beta | Lower bound | Upper bound | |||
(Constant) | -4.933E-15 | .161 |
| .000 | 1.000 | -.414 | .414 |
Zscore (VtB) | 5.007 | 1.156 | 5.007 | 4.330 | .007 | 2.034 | 7.979 |
Zscore (TeB) | -3.831 | 1.087 | -3.831 | -3.526 | .017 | -6.624 | -1.038 |
Zscore (MVRB) | -4.415 | 1.116 | -4.415 | -3.957 | .011 | -7.284 | -1.547 |
Zscore (LF/HFB) | 1.428 | .427 | 1.428 | 3.340 | .021 | .329 | 2.526 |
-.728 | .360 | -.728 | -2.023 | .099 | -1.653 | .197 | |
Zscore (Ti) | -4.037 | 1.097 | -4.037 | -3.681 | .014 | -6.856 | -1.218 |
Zscore (Te) | -4.194 | 1.237 | -4.194 | -3.391 | .019 | -7.374 | -1.014 |
Zscore (FR) | -5.953 | 1.815 | -5.953 | -3.280 | .022 | -10.617 | -1.288 |
Zscore (CC) | -.705 | .283 | -.705 | -2.492 | .055 | -1.431 | .022 |
Dependent variable: Zscore (LF/HF) | |||||||
Note: Zscore (VtB) – standardized score of the tidal volume in RR; Zscore (TeB) – standardized score of the duration of expiration; Zscore (MVRB) – standardized score of the respiratory minute volume; Zscore (LF/HFB) – standardized score of the ratio of low frequency power to high frequency;.Zscore (Te) – standardized score of the duration of expiration; Zscore (Ti) – standardized score of the duration of inspiration; Zscore (FR) – standardized score of the breathing rate; Zscore (Vt) – standardized score of the tidal volume; Zscore (CC) – standardized score of the duration of cardiac cycle. | |||||||
The residuals of the linear regression model satisfied the necessary conditions. The observed distribution exhibited a slight right skewness and a random scatter without any discernible pattern (Fig. 2). These characteristics indicate that the developed model is optimal for predicting LF/HF in individuals with abdominal breathing based on the provided data.
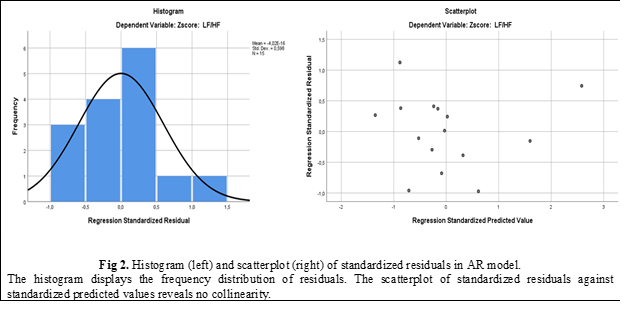
Discussions
The present study documents that PR parameters, measured during both resting breathing and abdominal breathing, can predict sympathovagal modulation in healthy individuals undergoing breathing pattern re-education. Based on the obtained results, we determined that Vt has the greatest predictive power for assessing the balance between sympathetic and parasympathetic activity, as measured by the LF/HF ratio in individuals practicing abdominal breathing. The unstandardized coefficient for Vt is 5.007, followed by Te (B = -3.831) and MVR (B = 4.415), both measured during resting breathing. Consequently, we can predict that decreasing Vt or increasing MVR during resting breathing may lead to a reduction in the LF/HF ratio during abdominal breathing. This can be explained by an accentuation of parasympathetic influences and a decrease in sympathetic influences. However, these findings are not immediately evident due to the general lack of change in HRV. Further studies incorporating longer periods of abdominal breathing may reveal more pronounced alterations in HRV.
The LF/HF ratio observed during the abdominal breathing pattern can also be predicted by the PR parameters measured during abdominal breathing. The most effective predictors are found to be the PR time parameters, including the frequency of breathing in the abdominal breathing pattern (FR) with a coefficient of -5.953, the duration of the inspiratory phase (Ti) with B = -4.037, and the duration of the expiratory phase (Te) with B = -4.194. Increasing FR along with an increase in Ti or increasing FR along with an increase in Te would lead to a reduction in the LF/HF ratio, thereby improving the sympathovagal balance.
Therefore, we can assume that individuals with higher MVR at rest and correspondingly higher frequency in abdominal breathing may experience a decrease in the sympathovagal balance during abdominal breathing.
In conclusion, by modulating these two parameters of the breathing pattern, namely MVR at rest and the total duration of a respiratory cycle (which influences the frequency of breathing), during normal breathing in healthy individuals, we can potentially enhance the sympathovagal balance.
Conclusion
The statistical analysis data presented in this study enable us to propose a hypothesis that certain volume and time parameters of the breathing pattern have the potential to predict changes in the ratio between sympathetic and vagal tone of the heart. Specifically, abdominal breathing has shown the ability to restore or normalize the sympathovagal balance by modulating the duration of inspiration or expiration.
To gain a deeper understanding of the practical applications of breathing pattern parameters in restoring the LF/HF ratio, particularly in disorders characterized by an elevated sympathovagal balance
Competing interests
None declared.
Patient consent
Obtained
Ethics approval
This study was approved by the Research Ethics Committee of Nicolae Testemițanu State University of Medicine and Pharmacy (minutes no. 15 from 11.01.2016).
Author’s ORCID ID
Andrei Ganenco – https://orcid.org/0000-0002-9835-5461
References
Strauss-Blasche G, Moser M, Voica M, McLeod DR, Klammer N, Marktl, W. Relative timing of inspiration and expiration affects respiratory sinus arrhythmia. Clin Exp Pharmacol Physiol. 2000 Aug;27(8):601-6. doi: 10.1046/j.1440-1681.2000.03306.x.
Lehrer PM, Smetankin A, Potapova T. Respiratory sinus arrhythmia biofeedback therapy for asthma: a report of 20 unmedicated pediatric cases using the Smetankin method. Appl Psychophysiol Biofeedback. 2000 Sep;25(3):193-200. doi: 10.1023/A:1009506909815.
Lehrer PM, Vaschillo E, Vaschillo B. Resonant frequency biofeedback training to increase cardiac variability: rationale and manual for training. Appl Psychophysiol Biofeedback. 2000 Sep;25(3):177-91. doi: 10.1023/A:1009554825745.
Draghici AE, Taylor JA. The physiological basis and measurement of heart rate variability in humans. J Physiol Anthropol. 2016 Sep;35(1):22 doi: 10.1186/s40101-016-0113-7.
Berntson GG, Bigger Jr JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997 Nov;34(6):623-48. doi: 10.1111/j.1469-8986.1997.tb02140.x.
Tiwari R, Kumar R, Malik S, Raj T, Kumar P. Analysis of heart rate variability and implication of different factors on heart rate variability. Curr Cardiol Rev. 2021;17(5):e160721189770. doi: 10.2174/1573403X16999201231203854.
Lehrer P. Biofeedback training to increase heart rate variability. In: Lehrer P, Woolfolk RL, Sim WE, editors. Principles and practices of stress management. 3rd ed. New York: Guilford Press; 2007. p. 227-48.
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93(5):1043-65. doi: 10.1161/01.CIR.93.5.1043.
Thomas BL, Claassen N, Becker P, Viljoen M. Validity of commonly used heart rate variability markers of autonomic nervous system function. Neuropsychobiology. 2019;78(1):14-26. doi: 10.1159/000495519.
Hopf HB, Skyschally A, Heusch G, Peters J. Low-frequency spectral power of heart rate variability is not a specific marker of cardiac sympathetic modulation. Anesthesiology. 1995 Mar;82(3):609-19. doi: 10.1097/00000542-199503000-00002.
Moak JP, Goldstein DS, Eldadah BA, Saleem A, Holmes C, Pechnik S, Sharabi Y. Supine low-frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Heart Rhythm. 2007 Dec;4(12):1523-9. doi: b10.1016/j.hrthm.2007.07.019.
Billman GE. Heart rate variability: a historical perspective. Front Physiol. 2011 Nov;2:86. doi: 10.3389/fphys.2011.00086.
Billman GE. The effect of heart rate of the heart rate variability response to autonomic interventions. Front Physiol. 2013 Aug 26;4:222. doi: 10.3389/fphys.2013.00222.
Reyes del Paso G, Langewitz W, Mulder LJM, van Roon A, Duschek S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology. 2013 May;50(5):477-87. doi: 10.1111/psyp.12027.
Haensch C-A, Lerch H, Jörg J, Isenmann S. Cardiac denervation occurs independent of orthostatic hypotension and impaired heart rate variability in Parkinson's disease. Parkinsonism Relat Disord. 2009 Feb;15(2):134-7. doi: 10.1016/j.parkreldis.2008.04.031.
RBI Instrumentation. VISURESP RBI-Instrumentation [Internet]. Meylan (France): RBI; 2005 [cited 2022 Apr 25]. Available from: https://rbi-instrumentation.monsite-orange.fr/page6/index.html.

