Introduction
Stenosis of the pyeloureteral junction (PUJ) is a condition that disrupts the urinary passage at the level of the renal pelvis and the upper third of the ureter. It occurs in 1 in 500 newborns, with a higher prevalence in males than females, with a ratio of 2:1 [1, 2]. Stenosis of the pyeloureteral junction is considered the most common cause of congenital hydronephrosis, and it is determined by anomalies in the anatomical structure of the renal-urinary tract, such as ureteral hypoplasia, high insertion of the ureter, aberrant vessels, and adhesions that compress and irritate the ureter, leading to urodynamic disorders and subsequent morphological changes. However, PUJ stenosis can also occur later in life as a secondary condition, with etiologies including: (a) extrinsic factors such as compression of the PUJ due to retroperitoneal fibrosis, retroperitoneal lymphadenopathy, retroperitoneal tumors; (b) intrinsic factors such as fibrosis of the PUJ caused by stones, chronic inflammation, exposure to radiation, radiotherapy, transitional cell and urothelial tumors of the ureter, and iatrogenic causes [3-5].
There is a variety of minimally invasive interventions available for the treatment of stenosis and strictures of the pyeloureteral junction (PUJ): balloon dilation, laparoscopic pyeloplasty, antegrade (percutaneous) endopyelotomy, and retrograde endopyelotomy. Although laparoscopic pyeloplasty is considered the gold standard for all causes of PUJ stenosis, endoscopic management can be considered as a first-line treatment for aperistaltic ureteral segment and intrinsic ureteral stenosis (<2 cm) in the absence of aberrant renal vessels [6]. Methods with maximal precision and direct ureteroscopic visualization of the lesion are preferred over blind incisions with Acucise, as they carry a higher risk of intraoperative bleeding [7, 8].
Ureteroscopic endopyelotomy with LASER was first described by Inglis and Tolley in 1986 [9]. Retrograde incision of PUJ offers several advantages: shorter procedure and recovery duration, reduced hospitalization time, minimal use of postoperative analgesics, and avoidance of external urinary drainage. Although it does not show more significant results compared to open surgeries, studies on the success rate of LASER endopyelotomy in patients with primary or secondary PUJ continue to this day. Literature results show a success rate of retrograde LASER endopyelotomy ranging from 39% to 100% in primary or secondary PUJ [10, 11]. Other studies present ureteroscopic LASER endopyelotomy as a treatment method for PUJ stenosis with a success rate starting at 89% [12, 13]. The primary objective of any intervention, whether open or minimally invasive, is to preserve renal function.
The purpose of presenting these cases is to evaluate the effectiveness and morbidity of retrograde endoscopic incision for restoring urinary passage in PUJ, as well as the duration of maintaining the postoperative result.
Case presentation
Inclusion and exclusion criteria, patients’ description, case history, investigation results.
Patient selection was based on specific indications for retrograde endopyelotomy, aiming to achieve a higher success rate in cases without significant hydronephrosis, with a ureteral segment affected by stenosis less than 2 cm in length, and lacking aberrant vasculature. The study data was collected by recording the clinical and paraclinical data of the enrolled subjects. Patients with extrinsic ureteral obstruction, stenotic segments exceeding 2 cm, severe hydronephrosis, renal function less than 20% of the affected kidney by PUJ stenosis, tumor involvement at the site of obstruction, high ureteral insertion, pediatric patients, and individuals whose treatment modality did not involve the employed method were excluded from the study.
From November 2022 to February 2023, a cohort of 5 female patients of 35 to 45 years of age with ureteropelvic junction stenosis were admitted for retrograde laser pyelotomy. All participants presented with ureteral stenosis and secondary PUJ obstruction resulting from chronic inflammation due to renal calculi that periodically lodges in PUJ. The primary symptoms reported by patients included lumbago, recurrent urinary tract infections, and, in one case, hematuria. Among the five patients, three exhibited grade 2 hydronephrosis, while the remaining two showed grade 1 hydronephrosis, as confirmed by ultrasonography. The renal function of the affected kidney was evaluated using scintigraphy, revealing a function greater than 30% in all candidates. The diagnosis was established based on computed tomography and intravenous pyelography, with the identification of contrast medium restriction at the junction without visualization of the ureter or encountering delayed passage (image of the distal portion of the ureter appear after 30 minutes). The length of the defect and the degree of hydronephrosis were determined using the aforementioned imaging methods. Renal function was assessed through dynamic scintigraphy. CT angiography was performed to exclude the presence of aberrant vessels.
The patients were prepared for the intervention by treating urinary tract infection, if present, performing ureteral stenting to facilitate drainage of infected urine, and resolving the renal calculi.
Treatment plan, surgical technique, and follow-ups
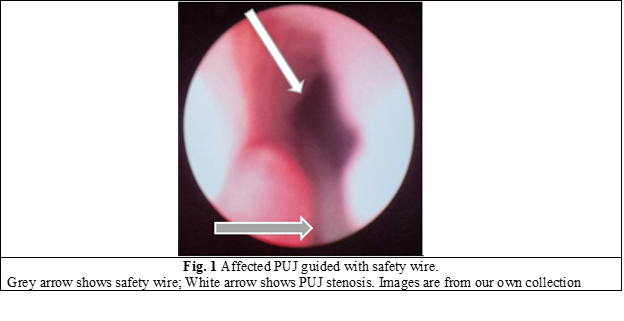
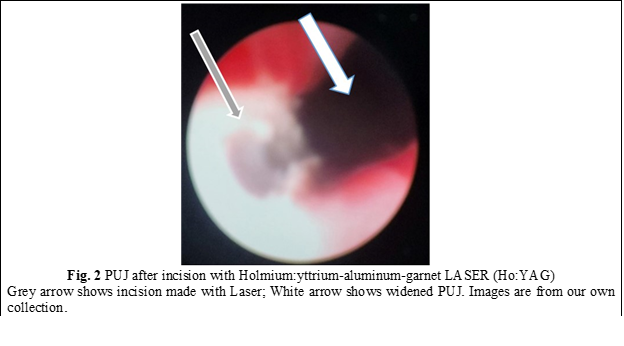
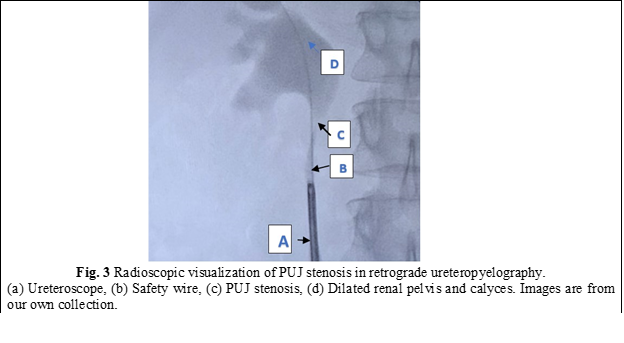
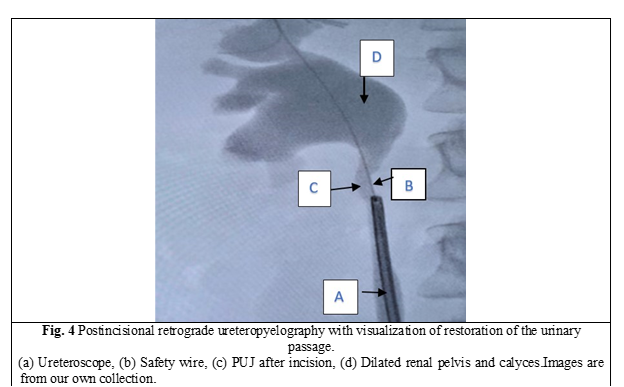
In all cases, a semirigid ureteroscope was employed for the procedure. In instances where the ureter presented excessive narrowness, impeding access to the upper third, a 5-Fr JJ stent was inserted for a duration of 2 weeks to facilitate ureteral dilation and establish an adequate working space. Subsequently, the patients were scheduled for stent extraction and the continuation of the previously determined treatment protocol. All patients underwent spinal anesthesia. Initially, intraoperatively, retrograde ureteropyelography was performed by injecting contrast medium into the ureter via the ureteroscope, allowing for repeated assessment of the anatomy of the PUJ stenosis. A semirigid ureteroscope, specifically the OES Pro 6.4/7.8 Fr x 430 mm model with a 4.2 Fr working channel, was utilized. The safety guide was used to reach the level of the pelviureteric junction stenosis as we can see in Fig. 1. A 365-µm laser fiber was employed during the procedure. Using real-time imaging guidance, the incision was made in a postero-lateral, caudo-cranial direction until visualization of the normal urothelium was achieved (Fig. 2), utilizing Ho:YAG LASER with an energy range of 0.5-3.5J and a frequency of 5-20. A control retrograde ureteropyelography was performed to determine the estimated length and level of the stenosis (Fig. 3). Post-incisional retrograde ureteropyelography was performed to assess the outcome (Fig. 4). Following the procedure, patients received the placement of 7-8 Fr JJ stents, which remained in place for a period of 4 weeks. After the completion of the procedure, patients were catheterized with a 16 Fr Foley catheter for 24 hours, following which they were discharged for outpatient follow-up and continuation of medical treatment at home. After a period of 3-4 weeks, patients were called back for reevaluation and analysis of postoperative results. During the first month, ultrasound, urine culture, and intravenous urography (IVU) were performed to assess urinary passage. If the positive result was maintained, the patient would be scheduled for a follow-up examination after 6 months. Successful treatment was defined by the resolution of symptoms, restoration of urinary passage, shortened visualization time of contrast in the ureter as observed through imaging studies, absence of obstructive patterns in dynamic scintigraphy, and preserved renal function. The half-life (T ½) of the radiopharmaceutical preparation (RFP) was less than 20 minutes. If these criteria were not met, the treatment was considered unsuccessful. Postoperative complications were classified according to the Clavien-Dindo classification system [14]. All patients have been informed about the surgical technique, purpose, and complications of the procedure. Consent for the surgical intervention and the use of personal data for the study has been obtained from each patient.
Results
Subjects involved in the present study were all detected with grade 2 (3 patients) and grade 1 (2 patients). When assessing pre-procedural (PUJ stenosis (Fig. 3)) and post-procedural results in retrograde ureteropyelography, we observe the restoration of urinary passage in all cases (Fig. 4). The average duration of the surgical intervention was 42.8 ± 2,58 minutes, and the mean duration of hospitalization was 4 ± 1.73 days. The patients were followed up for an average period of 8 weeks. One patient experienced a postoperative complication in the form of a urinary tract infection on the second day, which prolonged the hospital stay by an additional 4 days. During the one-month follow-up, a statistically significant difference was observed between patients with grade 1 hydronephrosis (complete restoration of urinary flow) and those with grade 2 hydronephrosis (faster contrast visualization in UIV) (p < 0.005). Furthermore, all patients reported the resolution of symptoms within three months of postoperative clinical monitoring.
Discussion
Open pyeloplasty represents the gold standard in the treatment of ureteropelvic junction (UPJ) stenosis, with a success rate ranging from 80% to 97% [15, 16]. However, due to its drawbacks, such as the need for a large surgical approach, lengthy recovery period, and high cost, alternative minimally invasive methods have been explored. Despite the lower postoperative success rate, these alternative approaches offer advantages in terms of cost, intraoperative complications, recovery time, and length of hospital stay.
Thorough preoperative evaluation and investigation of the patient allow for the selection of suitable candidates for minimally invasive interventions, which offer a high postoperative success rate. The European Association of Urology guidelines recommend laser endopyelotomy as the first-line treatment with a grade C recommendation. It is primarily applied in cases of intrinsic stenosis with a defect length of less than 2 cm, absence of a dilated pelvis, high ureteral insertion, renal split function less than 20%, and ipsilateral renal calculi. The level of evidence for this recommendation is 4 [17]. Some studies have reported a higher efficacy of laser endopyelotomy in cases of primary UPJ obstruction. Other authors did not observe a difference, while some reported a higher success rate in patients with secondary etiology of UPJ obstruction [11, 18]. Gupta and colleagues have reported on the success rate of retrograde endopyelotomy based on the degree of hydronephrosis and the length of the stenotic segment. In both cases, as the degree of hydronephrosis and the length of the stenotic segment increase, the success rate decreases. However, the difference in results between the two cases is not statistically significant [19]. Similarly, Rassweiler and colleagues have also addressed the correlation between multiple factors that influence the success rate, with one of the factors being hydronephrosis. They found that as the severity of hydronephrosis increases, the postoperative outcomes become less favorable [20], which is consistent with the findings of the current study. The complication rate is estimated to be between 5% and 35%, with the most common complication being urinary tract infection (UTI) [21]. In our case, the complication rate was 20%, with a UTI being identified in a patient on the second day postoperatively during a urine analysis. The duration of stent placement for ureteral integrity restoration post-endopyelotomy varies among different authors. Anil Mandhani and colleagues conducted an animal study that showed positive results with complete recovery after 2 weeks [22]. Geavlete reported a necessary period of 6 weeks, as the urothelium regenerates in 5 days and the muscular tissue in 6 weeks [23]. In the current study, the stent was left in place for 4 weeks. The success rate in our study, at an 8-week patient follow-up, is currently 99.9%. However, patients require ongoing monitoring to assess the maintenance of JPU patency. Nevertheless, although the effectiveness of the intervention is continuously studied, JPU patency can decrease over time, as mentioned by Shalhav Al and colleagues in 1989 [24].
Conclusion
Compared to other minimally invasive methods such as antegrade endopyelotomy, retrograde incision with balloon (Acucise), laparoscopic, and robot-assisted procedures, the retrograde technique has several advantages, with cost-effectiveness being the most significant one: comparatively affordable equipment, shorter recovery period, and hospital stay. To achieve a clear success rate, certain factors need to be considered: defect length <2 cm, insignificant hydronephrosis, absence of a large pelvis, and high ureteral insertion, with preserved ipsilateral renal function. The current study results demonstrate an efficacy rate of 99.9% in the early months of monitoring. However, longer monitoring with a larger patient sample is necessary to provide more conclusive data in the future.
Competing interests
The authors report no conflicts of interest in this work.
Patient consent
Obtained.
Funding Statement
The authors report no financial support.
Authors’ contributions
VC conceived and designed the study, performed surgical interventions, collected and analyzed data, and significantly contributed to manuscript writing and revision. EP assisted in study design, participated as an assistant in surgical interventions, contributed to data analysis, and played a substantial role in manuscript drafting and critical review. AM assisted in study design, participated as an assistant in surgical interventions, provided clinical insights, and contributed to manuscript revision. CM assisted with data collection, participated as an assistant in surgical interventions, and participated in manuscript revision. Final manuscript was read and approved by all authors.
Authors’ ORCID IDs
Vladimir Caraion - https://orcid.org/0009-0003-2917-9665
Eduard Pleșca - https://orcid.org/0000-0002-0021-0396
Andrei Mezu - https://orcid.org/0009-0006-7355-9982
Corneliu Maximciuc - https://orcid.org/0009-0000-3163-8822
References
1.
European Association of Urology. EAU Guidelines on paediatric urology [Internet]. Arnhem: EAU; 2023 [cited 2023 May 12]. Available from: https://uroweb.org/guidelines/paediatric-urology/chapter/the-guideline.
Termos S, AlKabbani M, Ulinski T, Sanjad S, Kotobi H, Chalard F, et al. Ureteropelvic junction obstruction and parathyroid adenoma: coincidence or link? Case Rep Nephrol. 2017;2017:9852912. doi: 10.1155/2017/9852912.
Borin JF. Ureteropelvic junction obstruction in adults. Rev Urol. 2017;19(4):261-4. doi: 10.3909/riu0781.
Grasso M, Caruso RP, Phillips CK. UPJ obstruction in the adult population: are crossing vessels significant? Rev Urol. 2001;3(1):42-51.
Jackson L, Woodward M, Coward RJ. The molecular biology of pelvi-ureteric junction obstruction. Pediatr Nephrol. 2018;33(4):553-71. doi: 10.1007/s00467-017-3629-0.
Sutherland DE, Jarrett TW. Surgical options in the management of ureteropelvic junction obstruction. Curr Urol Rep. 2009;10(1):23-8. doi: 10.1007/s11934-009-0006-y.
Gerber GS, Kim JC. Ureteroscopic endopyelotomy in the treatment of patients with ureteropelvic junction obstruction. Urology. 2000;55(2):198-202; discussion 202-3. doi: 10.1016/s0090-4295(99)00530-0.
Kartal I, Cimen S, Karakoyunlu N, Sandikci F, Eraslan A, Yalcinkaya F. Factors affecting the effectiveness and success of retrograde holmium laser endopyelotomy as the primary treatment of ureteropelvic junction obstruction in adults. Urologia. 2021;88(1):34-40.doi: 10.1177/0391560320904259.
Sen H, Bayrak O, Erdem Yilmaz A, Turgut O, Ozturk M, Erturhan S, et al. Evaluation of the results of laser endopyelotomy with two different technique in ureteropelvic junction obstruction. J Pediatr Urol. 2021;17(3):397e1-e6. doi: 10.1016/j.jpurol.2021.01.035.
Braga LH, Lorenzo AJ, Skeldon S, Dave S, Bagli DJ, Khoury AE, et al. Failed pyeloplasty in children: comparative analysis of retrograde endopyelotomy versus redo pyeloplasty. J Urol. 2007;178(6):2571-5; discussion 2575. doi: 10.1016/j.juro.2007.08.050.
Stilling NM, Jung H, Norby B, Osther SS, Osther PJ. Retrograde ureteroscopic holmium laser endopyelotomy in a selected population of patients with ureteropelvic junction obstruction. Scand J Urol Nephrol. 2009;43(1):68-72. doi: 10.1080/00365590802473164.
Gnessin E, Yossepowitch O, Holland R, Livne PM, Lifshitz DA. Holmium laser endoureterotomy for benign ureteral stricture: a single center experience. J Urol. 2009;182(6):2775-9. doi: 10.1016/j.juro.2009.08.051.
Hibi H, Ohori T, Taki T, Yamada Y, Honda N. Long-term results of endoureterotomy using a holmium laser. Int J Urol. 2007;14(9):872-4. doi: 10.1111/j.1442-2042.2007.01835.x.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-13.doi: 10.1097/01.sla.0000133083.54934.ae.
Rassweiler JJ, Gozen AS, Erdogru T, Sugiono M, Teber D. Ureteral reimplantation for management of ureteral strictures: a retrospective comparison of laparoscopic and open techniques. Eur Urol. 2007;51(2):512-22; discussion 522-3. doi: 10.1016/j.eururo.2006.08.004.
Simforoosh N, Basiri A, Tabibi A, Danesh AK, Sharifi-Aghdas F, Ziaee SA, et al. A comparison between laparoscopic and open pyeloplasty in patients with ureteropelvic junction obstruction. Urol J. 2004;1(3):165-9.
Herrmann TR, Liatsikos EN, Nagele U, Traxer O, Merseburger AS; Eau Guidelines Panel on Lasers, Technologies. EAU guidelines on laser technologies. Eur Urol. 2012;61(4):783-95. doi: 10.1016/j.eururo.2012.01.010.
Seveso M, Giusti G, Taverna G, Piccinelli A, Benetti A, Maugeri O, et al. Retrograde endopyelotomy using the holmium laser: technical aspects and functional results. Arch Ital Urol Androl. 2005;77(1):10-2.
Gupta M, Tuncay OL, Smith AD. Open surgical exploration after failed endopyelotomy: a 12-year perspective. J Urol. 1997;157(5):1613-8; discussion 1618-9.
Rassweiler JJ, Subotic S, Feist-Schwenk M, Sugiono M, Schulze M, Teber D, et al. Minimally invasive treatment of ureteropelvic junction obstruction: long-term experience with an algorithm for laser endopyelotomy and laparoscopic retroperitoneal pyeloplasty. J Urol. 2007;177(3):1000-5. doi: 10.1016/j.juro.2006.10.049.
Elmussareh M, Traxer O, Somani BK, Biyani CS. Laser endopyelotomy in the management of pelviureteric junction obstruction in adults: a systematic review of the literature. Urology. 2017;107:11-22. doi: 10.1016/j.urology.2017.04.018.
Mandhani A, Kapoor R, Zaman W, Kumar A, Bhandari M, Gambhir S. Is a 2-week duration sufficient for stenting in endopyelotomy? J Urol. 2003;169(3):886-9. doi: 10.1097/01.ju.0000051341.22163.21.
Geavlete P, Georgescu D, Mirciulescu V, Nita G. Ureteroscopic laser approach in recurrent ureteropelvic junction stenosis. Eur Urol. 2007;51(6):1542-8. doi: 10.1016/j.eururo.2006.08.035.
Shalhav AL, Giusti G, Elbahnasy AM, Hoenig DM, McDougall EM, Smith DS, et al. Adult endopyelotomy: impact of etiology and antegrade versus retrograde approach on outcome. J Urol. 1998;160(3 Pt 1):685-9. doi: 10.1016/S0022-5347(01)62755-1

