Introduction
Intestinal anastomosis dehiscence is a surgical complication that remains an important issue in modern public health and has a major medical, social and economic impact. Anastomosis dehiscence can be considered one of the quality indices of medical care in surgical departments [1-3]. According to medical literature, the incidence of the development of colon anastomosis dehiscence ranges widely, from 3.3% to 25.1% [4, 5]. The occurrence of this complication is associated with a significant increase in postoperative morbidity and mortality [6], and, respectively, reflects the quality of the surgical service [7].
Currently, in order to protect the intestinal anastomosis and prevent the occurrence of anastomosis dehiscence, various studies are carried out based on studying the role of human blood elements and the use of various synthetic substances. A new proposed method is the use of platelet-rich plasma. This term was first proposed in 1998 by Marx [8]. Platelet-rich plasma ensures the penetration of platelets in excessive quantity, accelerating the wound healing process. The regenerative effect can be explained by modulating growth factors such as platelet-derived growth factor, insulin-like growth factor, transforming growth factors β1 and β2 [9]. The active secretion of these factors is initiated due to the blood coagulation process and begins within 10 minutes of coagulation. More than 95% of growth factors are synthesized during the first hour [10]. Clinical and experimental studies are necessary for the correct assessment of the effectiveness of the use of platelet-rich plasma in the local protection of the anastomotic area.
The study objective was to assess the efficacy of platelet-rich plasma in local protection of colon anastomosis.
Material and methods
Experimental part. Forty-two rats were divided into two groups: group I – unprotected colon anastomosis (n = 21); group II – protected colon anastomosis with local application of platelet-rich plasma (n = 21). Anesthesia was performed by intraperitoneal administration of ketamine hydrochloride solution (Kalypsol®, Gedeon Richter, Hungary). The experimental study was carried out in accordance with the „Directive 2010/63/EU of the European Parliament and of the Council” regarding the protection of animals used for scientific purposes [11]. The research project was examined at the meeting of the Nicolae Testemiţanu State University of Medicine and Pharmacy Research Ethics Committee, which took place on September 15, 2014.
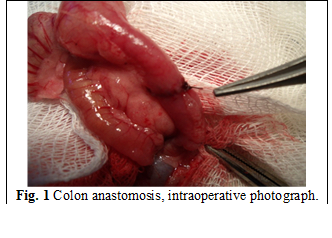
Colon anastomosis was performed according to the standardized method, which included the following steps: opening the abdominal cavity through mid-median laparotomy; transection of the transverse colon at a distance of 1 cm from the cecum with the application of end-to-end unprotected colon anastomosis with continuous suture, using Polypropylene monofilament thread 5/0 in rats from group I (Fig. 1). In rats from group II, platelet-rich plasma was applied on the line of anastomosis. Layered closure of the abdominal wall was performed.
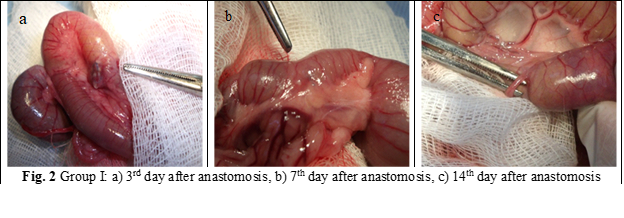
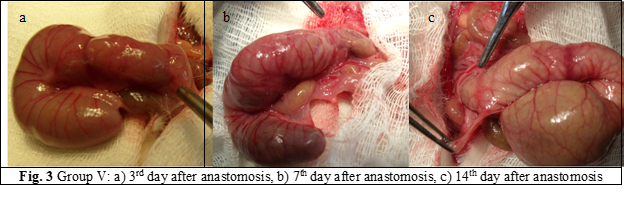
Animals were euthanized in CO2 chamber. The autopsy of the rats was performed at 3rd, 7th and 14th postoperative day, 7 rats for each group. The anastomoses were examined macro- and microscopically to assess for abscesses, dehiscence, signs of peritonitis, adherences and to evaluate mechanical resistance. During autopsy, a 4 cm portion of the colon was taken, with the suture in the center and 2 cm on both sides of the anastomosis (fig. 2 a, b, c; fig. 3 a, b, c). The fragments were prepared for histological examination and to evaluate the anastomosis burst pressure.
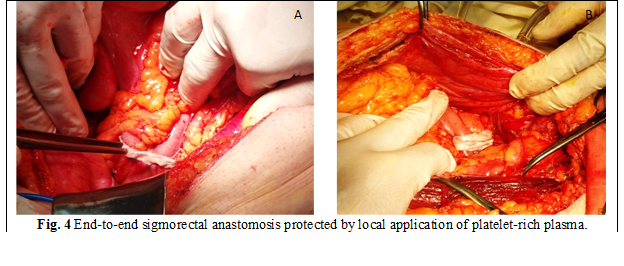
Clinical part. 37 patients were included in the clinical study, who underwent colon anastomosis. The patients were divided into 2 groups: group I (n = 16) had unprotected colon anastomosis and group II (n = 21) - protected anastomosis with platelet-rich plasma. The anastomosis was applied in 2 steps: internal with suture polydioxanone 3/0 - 4/0 and external - polypropylene 3/0 (fig. 4 A, B).
The patients were carefully monitored in the postoperative period. Procalcitonin was used for the laboratory diagnosis of colon anastomosis dehiscence. Postoperatively, the changes in the level of serum procalcitonin on the 3rd, 5th and 7th day were studied. For the assessment of procalcitonin, an immunoenzymatic analysis kit (Вектор Б, Novosibirsk, Russia) was used performed on the automated immunological ELISA analyzer Uno (Human), Germany. The normal value of procalcitonin is < 0.1 ng/ml.
Results
Experimental study. Analyzing the degree of adhesion formation according to van der Hamm's score [12], we can conclude that there is a statistically insignificant increase in the degree of intra-abdominal adhesion formation from the 3rd to the 7th day, with a statistically insignificant decrease from 7th to 14th day.
According to the data obtained in the current study, a statistically insignificant increase in the degree of adhesion formation was demonstrated in group II vs. group I (NS).
The burst pressure of the anastomosis was also studied. According to the obtained data, there is a statistically significant increase in the burst pressure of the anastomosis from the 3rd to the 7th postoperative day and a statistically insignificant decrease of this parameter from the 7th to the 14th postoperative day (Fig. 5).
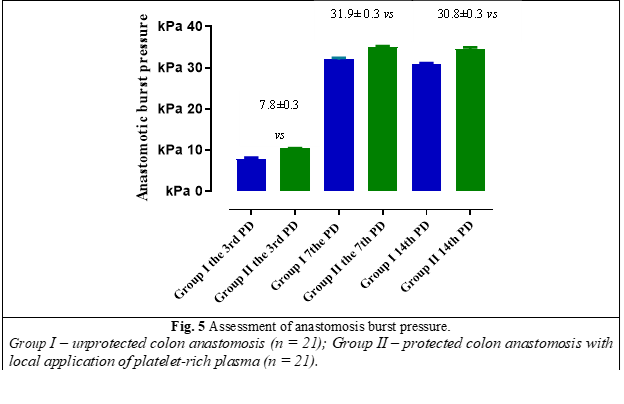
Analyzing the obtained data, a statistically significant increase (p ˂ 0.05) of the anastomosis burst pressure was demonstrated on the 3rd, 7th and 14th postoperative day in group II vs group I.
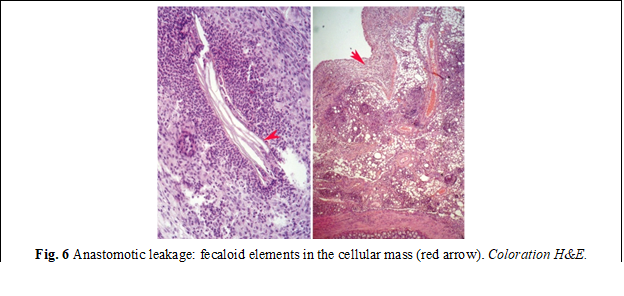
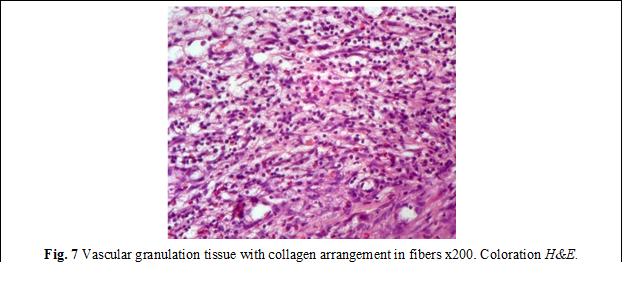
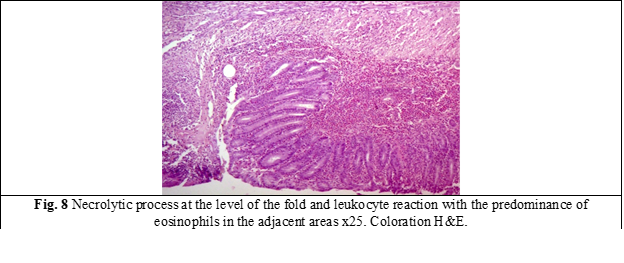
Histological examination. Microscopic examination of the samples from group I revealed deformations and volume changes, reactive edema, adhesions and slowing of the regenerative processes. An important key in this group was the activation of the local bacterial microflora, which on the 3rd and 7th day, due to its proteolytic features, manifested itself through excessive activity, forming the bacterio-necrotic-purulent demarcation line. In the anastomoses with a predominance of bacterial flora, the necrolytic and inflammatory processes in some places were significantly more aggressive, sometimes with penetration into the depth of the anastomosis, thus contributing to the appearance of anastomotic leakages, abscesses, deformations of the anastomosis, as well as the appearance of diverticula (fig. 6). The formation of granulation tissue was manifested by increased proliferation of fibroblasts and the presence of collagen deposits (fig. 7). Persistence of dystrophy of the ganglioneuronal structures of the Auerbach plexus was frequently detected, at a distance of up to 2.5 cm from the anastomosis.
The microscopic examination of the samples from group II demonstrated that in most cases the anastomoses had a tubular appearance, with preserved permeability. The histological examinations in this group were demonstrated by the significant regenerative processes.
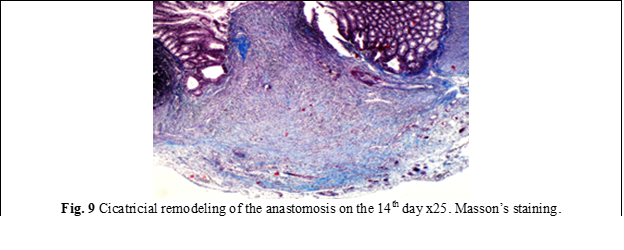
In group II, in the internal area of the anastomosis was noticed a decrease in the activity of thrombo-vascular and exudative processes, while in the external area these processes were absent, unlike group I (fig. 8). From the external examination, a mantle of newly formed tissue with an insignificant tissue-tuberous appearance was observed, prominent outside the anastomotic area with the activity of subtotal/total epithelization attested on the 14th day. In group II, it was attested a numerical increase of mast cells from 2-3 to 8-9 at x20 high-power field, mainly in the external area of the anastomosis. In parallel, the emphasis of the proliferative-fibroblastic process was observed at the level of the cellular-adipose tissue, directed towards the anastomosis, with voluminous hyper granulated mast cells present or with the spread of granules in the extracellular matrix. Compared to the anastomoses in group I, the dystrophy of the nerve plexuses was insignificant, with the exception of 2 cases. It is necessary to mention that no anastomotic leakages were detected in this group. On the 14th day, the line of anastomosis in some places was completely diminished macroscopically (Fig. 9).
According to this study, the mast cells were actively involved in the initial stages of triggering the acute inflammatory process. The attested cellular morphological manifestations were characterized by hypergranulation, which reflects the activation of mast and degranulation cells, the phenomenon of release in the extracellular matrix of mediators and chemotactic substances [13, 14], which contribute to the initiation and migration of leukocytes towards the anastomosis areas. It is also necessary to mention that the degranulation phenomenon reflects the activation of the neovascularization process through the release of endothelial-vascular growth factors and platelet activation. Based on the experimental data, we can conclude that the application of platelet-rich plasma on the anastomosis line does not worsen the adhesion process and increases statistically significant the burst pressure of the colonic anastomosis. The microscopical examination showed the acceleration of regenerative processes, in particular, angiogenesis and fibrillogenesis, in group II vs. group I (p < 0.05).
Clinical study. Thirty-seven patients were included in the clinical study, who were hospitalized urgently or electively in the Institute of Emergency Medicine. The average age was 59.49 ± 2.16 (23-78) years. There were 21 males (56.75%), and 16 females (43.24%), the ratio M:F = 1.3:1. 16 patients were included in group I, of which 10 – males and 6 – females; in group II – 11 males and 10 females. The distribution of patients by sex and age is shown in table 1.
Table 1. Distribution of patients according to age | |||||||
Age (years) | 20-30 | 31-40 | 41-50 | 51-60 | 61-70 | 71-80 | Total |
Males | 1 (%) | 4 (%) | - | 7 (%) | 7 (%) | 2 (%) | 21 (%) |
Females | - | - | 1 (%) | 2 (%) | 9 (%) | 4 (%) | 16 (%) |
Total | 1 (%) | 4 (%) | 1 (%) | 9 (%) | 16 (%) | 6 (%) | 37 (%) |
According to the etiology of the pathological process, the most predominant cause was neoplasia. Colon cancer was diagnosed in 17 cases (45.94%), other localized cancer - 2 (5.4%) cases, terminal colostomy – 7 (18.9%) cases, terminal ileostomy – 4 (10.8%) cases, colonic fistula – 2 (5.4%) cases, adhesion disease - 1 (2.7%) case, appendicular plastron - 1 (2.7%) case, appendicular mucocele - 1 (2.7%) case, sigmoid diverticulum - 1 (2.7%) case, colonic endometriosis - 1 (2.7%) case.
There were miscellaneous types and indications of surgeries. In 10 cases - the surgical procedures were performed urgently, while in 27 cases – planned. In group I, right hemicolectomy was performed - 7 cases, colostomy reversal - 4 cases, ileostomy reversal - 3 cases, ileum resection with ileocolic anastomosis - 1 case, ileosigmoid bypass - 1 case. In group II, right hemicolectomy was performed - 6 cases, segmental colectomy - 4 cases, left hemicolectomy - 4 cases, colostomy reversal - 4 cases, subtotal colectomy - 2 cases, previous rectal resection - 1 case.
Currently, there are numerous definitions and classifications of intestinal anastomosis dehiscence. This study used the classification of anastomotic dehiscence proposed by the International Rectal Cancer Study Group in 2013 [15]. In group I, grade B anastomosis dehiscence was diagnosed in 2 cases after planned right hemicolectomy, in one case after colostomy and one after ileostomy reversal. Grade C anastomotic dehiscence was detected in one case after emergency right hemicolectomy. This patient required repeated surgery – relaparotomy with anastomosis resection and ileostomy. In group II, there were no cases of anastomosis dehiscence. Thus, from the results of the clinical study data, the incidence of anastomotic dehiscence was in group I – 4 (25%) cases of grade B and 1 (6.25%) of grade C vs. group II – 0 cases (p = 0.01).
In the postoperative period, procalcitonin level was evaluated. Based on the obtained data, in uncomplicated cases with the development of anastomotic dehiscence, there is an increase in the serum level of procalcitonin on the 3rd postoperative day with a subsequent decrease on the 5th and 7th postoperative day. During the progressing of the colon anastomotic dehiscence a statistically significant increase in the procalcitonin serum level is determined, in particular - in grade C anastomotic dehiscence, on the 3rd, 5th and 7th postoperative day (р < 0.0001).
Discussions
Colorectal surgery represents an important field of contemporary surgery, due to the increasing incidence of surgical pathology of the colon. Despite the breakthroughs of modern medicine in general, and surgery in particular, dehiscence of intestinal anastomosis was and remains one of the most dangerous postoperative complications [16], without significant improvements [5].
Currently there are different definitions of intestinal anastomosis dehiscence. The Surgical Infection Study Group in 1991 defined anastomosis dehiscence as the leakage of intestinal contents through the surgical connection between two cavity organs [17]. According to the results from Komen N. et al., anastomosis dehiscence represents the leakage of intestinal content into the peritoneal cavity through the defect of anastomosis [18]. According to other authors, anastomosis dehiscence can be defined as the defect of the intestinal wall, which leads to the communication between the intra- and extraluminal compartment [19]. The International Study Group of Rectal Cancer defines dehiscence of the intestinal anastomosis as a communication between the intra- and extraluminal compartment through an anastomosis defect in the intestinal wall between the colon and the rectum or between the colon and anus. According to the data of this group, the abscess near the anastomosis, even without an obvious fistula, is to be interpreted as anastomotic dehiscence [15].
Early diagnosis and timely surgical intervention have a considerable influence on the final result. The diagnosis is primarily based on clinical data; however, epidural block [20], administration of analgesic drugs, including opioids, antibacterial therapy, and infusion therapy can reduce the clinical signs and symptoms. The increase in the concentration of serum inflammatory markers can be suggestive for anastomosis dehiscence, but the accuracy and specificity of the method is low, and the respective changes appear with a significant delay. The role of procalcitonin as a serological marker of intestinal anastomosis dehiscence is currently being researched. According to literature data, the increase in the serum concentration of procalcitonin was detected in all patients undergoing colorectal interventions, without complications, on the first postoperative day, with subsequent normalization on the 4th postoperative day in patients without complications and with a statistically significant increase on the 3rd to 5th postoperative day in patients with major anastomotic dehiscence [21].Mokart et al. demonstrated the increased sensitivity and specificity of procalcitonin regarding the early diagnosis of septic complications after oncological surgery [22]. Thus, procalcitonin can be used as an early serological marker of major anastomotic dehiscence.
Protecting intestinal anastomosis remains an important problem in colorectal surgery. Various research is currently being carried out focusing on studying the natural factors that influence the regenerative processes. A new method in this field is the local application of platelet-rich plasma.Currently, platelet-rich plasma is used in various fields of contemporary medicine, such as: periodontology [23], maxillofacial surgery [24], dental implantology [25], orthopedics and sports medicine [26], chronic skin ulcers [27]. In the specialized literature, there are case reports about the use of platelet-rich plasma in colorectal surgery. Yol S. et al. (2008) experimentally proved that the application of platelet-rich plasma on colonic anastomosis suture is associated with an increase in the burst pressure of the anastomosis, by increasing the tissue hydroxyproline concentration [28]. Microscopical examinations of the anastomoses protected with platelet-rich plasma demonstrated the improvement of regenerative processes, in particular, neoangiogenesis and fibrillogenesis. Therefore, the local application of platelet-rich plasma on the anastomosis suture does not significantly worsen the abdominal adhesion process, statistically increases the burst pressure of the anastomosis and increases the regenerative processes.
Thus, the use of platelet-rich plasma has a beneficial influence on the healing process of the colon anastomosis and improves postoperative results.
Conclusions
A statistically significant increase (p ˂ 0.05) in anastomotic burst pressure was demonstrated on the 3rd, 7th and 14th postoperative day in group II vs. group I. Also, the use of platelet-rich plasma does not significantly influence the abdominal adhesion process.
The microscopical examination demonstrated that the local use of platelet-rich plasma leads to the improvement of regeneration processes in the anastomosis area, in particular, neoangiogenesis and fibrillogenesis (p < 0.05).
The use of platelet-rich plasma for local protection of colonic anastomosis improves postoperative outcomes.
Competing interests
None declared.
Patient consent
Obtained.
Ethical Statement
This study was carried out in accordance with the European Convention for the Protection of Vertabrate Animals Used for Experimental and Other Scientific Purposes („Directive 2010/63/EU of the European Parliament and of the Council”) and approved by the Research Ethics Committee of Nicolae Testemiţanu State University of Medicine and Pharmacy, Minutes No. 5 from 15.09.2014.
Author’s ORCID ID
Elena Pleșco – https://orcid.org/0000-0001-6779-2282
References
Essani R, Bergamaschi R. Anastomotic leak in colorectal surgery: a review. Gastroenterol Pol. 2009;16(2):123-127.
Lodhi FB, Shafiq M, Farooq T, Hussain R. Anastomotic leak after small gut surgery. Professional Med J Mar. 2006;13(1):47-50.
Thompson GA, Cocks JR, Collopy BT, Cade RJ, Ewing HP, Rogerson JW, Turner PL. Colorectal resection in Victoria: a comparision of hospital based and individual audit. Aust N Z J Surg. 1996;66(8):520-524.doi: 10.1111/j.1445-2197.1996.tb00801.x.
Bondar’ GV. Profilaktika nesostoiatel’nosti anastomoza pri khirurgicheskom lechenii bol’nykh oslozhnennym rakom tolstoi kishki [Prevention of anastomotic leakage during surgical treatment of patients with complicated colon cancer]. [Vestnik Hyg Epidemiol]. 2001;5(1):103-107. Russian.
Kecherukov AI, Chernov IA, Giunter VE, Aliev FSh. Sposob formirovaniia kompressionnogo terminal’nogo tolstokishechnogo anastomoza. [Method for forming a compression terminal colonic anastomosis]. [Surgery]. 2005;11(2):64-67. Russian.
Post IL, Verheijen PM, Pronk A, Siccama I, Houweling PL. Intraoperative blood pressure changes as a risk factor for anastomotic leakage in colorectal surgery. Int J Colorectal Dis. 2012;27(6):765-772. doi: 10.1007/s00384-011-1381-7.
Agaba AE, Duthie GS. Anastomotic leakage: experience from a colorectal unit. Nig J Surg Research. 2004;6(1-2):49-52. doi: 10.4314/njsr.v6i1-2.54793.
Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(6):638-646. doi: 10.1016/s1079-2104(98)90029-4.
Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sitis for implants. Int J Oral Maxillofac Implants. 1999;14(4):529-535.
Kiran NK, Mukunda KS, Tilak Raj TN. Platelet concentrates: a promising innovation in dentistry. J Dental Sci Res. 2011;2(1):50-61.
Hartung T. Comparative analysis of the revised Directive 2010/63/EU for the protection of laboratory animals with its predecessor 86/609/EEC – at 4 report. ALTEX. 2010;27(4):285-303. doi: 10.14573/altex.2010.4.285.
van der Ham AC, Kort WJ, Weijma IM, van den Ingh HF, Jeekel H. Effect of antibiotics in fibrin sealant on healing colonic anastomoses in the rat. Br J Surg. 1992;79(6):525-8. doi: 10.1002/bjs.1800790617.
Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory di seases end the effect of acute stress. J Neuroimumunol. 2004;146(1-2):1-12. doi: 10.1016/j.jneuroim.2003.10.041.
Leal-Berumen I, Conlon P, Marshall JS. IL-6 production by rat peritonial mast cells is not becesasrily preceded by histamine release and can be induced by bacterial lipopolisaccharide. J Immunol. 1994;152(11):5468-5476.
Kulu Y, Ulrich A, Bruckner T, Contin P, Welsch T, Rahbari NN, Buchler MW, Weitz J. Validation of the International Study Group of Rectal Cancer definition and severity grading of anastomotic leakage. Surgery. 2013;153(6):753-761. doi: 10.1016/j.surg.2013.02.007.
Ibragimov R. Opyt primeneniia bioeksplantata na osnove modifitsirovannoi gialuronovoi kisloty dlia profilaktiki nesostoiatel’nosti anastomozov polykh organov (eksperimental’noe issledovanie) [Experience of using a bioexplant based on modified hyaluronic acid for the prevention of anastomotic failure of hollow organs (experimental study)]. [Bull Siberian Branch Rus Acad Med Sci]. 2009;6(140):19-23. Russian.
Peel AL, Taylor EW. Proposed definitions for the audit of postoperative infection: a discussion paper. Surgical Infection Study Group. Ann R Coll Surg Engl. 1991;73(6):385-388.
Komen N, Morsink MC, Beiboer S, Miggelbrink A, Willemsen P, van der Harst E, Lange JF, van Leeuwen WB. Detection of colon flora in peritoneal drain fluid after colorectal surgery: can RT-PCR play a role in diagnosing anastomotic leakage? J Microbiol Method. 2009;79(1):67-70. doi: 10.1016/j.mimet.2009.08.004.
Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, Laurberg S, den Dulk M, van de Velde C, Büchler MW. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the international study group of Rectal Cancer. Surgery. 2010;147(3):339-51. doi: 10.1016/j.surg.2009.10.012.
Pedersen EM, Qvist N, Bisgaard C, Kelly U, Bernhard A, Moller Pedersen S. Peritoneal microdialysis. Early diagnosis of anastomotic leakage after low anterior resection for rectosigmoid cancer. Scand J Surg. 2009;98(3):148-154. doi: 10.1177/145749690909800304.
Garcia-Granero A, Frasson M, Flor-Lorente B, Blanco F, Puga R, Carratala A, Garcia-Granero E. Procalcitonin and C-reactive protein as early predictors of anastomotic leak in colorectal surgery: a prospective observational study. Dis Colon Rectum. 2013;56(4):475-483. doi: 10.1097/DCR.0b013e31826ce825.
Mokart D, Merlin M, Sannini A, Brun JP, Delpero JR, Houvenaeghel G, Moutardier V, Blache JL. Procalcitonin, interleukin 6 and systemic inflammatory response syndrome (SIRS): early markers of postoperative sepsis after major surgery. Br J Anaesth. 2005;94(6):767-773. doi: 10.1093/bja/aei143.
Pradeep AR, Rao NS, Agarwal E, Bajaj P, Kumari M, Naik SB. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of 3-wall intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol. 2012;83(12):1499-1507. doi: 10.1902/jop.2012.110705.
Sammartino G, Dohan Ehrenfest DM, Carile F, Tia M, Bucci P. Prevention of hemorrhagic complications after dental extractions into open heart surgery patients under anticoagulant therapy: the use of leukocyte- and platelet-rich fibrin. J Oral Implantol. 2011;37(6):681-690. doi: 10.1563/AAID-JOI-D-11-00001.
Mazor Z, Horowitz RA, Del Corso M, Prasad HS, Rohrer MD, Dohan Ehrenfest DM. Sinus floor augmentation with simultaneous implant placement using Choukroun's platelet-rich fibrin as the sole grafting material: a radiologic and histologic study at 6 months. J Periodontol. 2009;80(12):2056-2064. doi: 10.1902/jop.2009.090252.
Zumstein MA, Berger S, Schober M, Boileau P, Nyffeler RW, Horn M, Dahinden CA. Leukocyte- and platelet-rich fibrin (L-PRF) for long-term delivery of growth factor in rotator cuff repair: review, preliminary results and future directions. Curr Pharm Biotechnol. 2012;13(7):1196-1206. doi: 10.2174/138920112800624337.
Cieslik-Bielecka A, Choukroun J, Odin G, Dohan Ehrenfest DM. L-PRP/L-PRF in esthetic plastic surgery, regenerative medicine of the skin and chronic wounds. Curr Pharm Biotechnol. 2012;13(7):1266-1277. doi: 10.2174/138920112800624463.
Yol S, Tekin A, Yilmaz H, Kucukkartallar T, Esen H, Caglayan O, Tatkan Y. Effects of platelet rich plasma on colonic anastomosis. J Surg Research. 2008;146(2):190-194. doi: 10.1016/j.jss.2007.05.015.