Introduction
The first trimester of gestation is the most complicated period, determining the normal course and outcome of pregnancy. This includes 3 critical periods: implantation, organogenesis, and placentation. The stage of implantation site formation in the endometrium and placentation results from the vascularization process induction of the chorionic plate and villi with the invasion of the trophoblast into the implantation "window" in the endometrial decidua, growth, and organization of the vascular network in the choriovillous stroma.
Early placentation disturbance contributes to morpho-functional changes in the gestational and germinal sites, followed by impaired maternal-embryo-fetal metabolic exchange during the establishment of hemochorionic blood circulation, manifested by primary placental insufficiency.
According to literature data, within placental insufficiency, the primary form is included [1], the cause of most early-term pregnancies such as a miscarriage or stagnant evolving pregnancies [2]. It is known that major reproductive disorders occur early in pregnancy [3], and deficiencies in vasculogenesis and angiogenesis in the chorionic villi are components of primary placental insufficiency, evolving through the dysfunction of uteroplacental or placento-fetal hemodynamics [4]. In this light, a number of studies have aimed to assess vascularization in the first trimester of gestation of pregnancies managed by medical abortion [5-8]. These studies have shed light on the developmental peculiarities of vascular circulation through angiogenesis, as well as vascularization disturbances in abortive disease [8], which are important steps in hemochorionic circulation stabilization [9]. Lisman et al. evaluated the peculiarities of the architectural vasculature in the normal and the hollow germinal sac of the hydatidiform mole using confocal scanning microscopy [6]. The authors have mentioned the impact of vascular-syncytial membranes formation and observed the reduction of endothelial cords with the maturation of pregnancy [6, 7].
Thus, from the 3rd week of intrauterine development, with the vascularization of the mesenchymal stroma (autochthonous vascularization), the period of placentation evolving during the first 12 w.g. begins. At the same time, there is an increase in the vessels of the allantois with the onset of embryo-hemochorionic circulation, determined by the presence of vascularization in the chorionic plate and villi [10]. In this context, the placenta is an important connecting organ in the maternal-placental-fetal system, which carries out the transport of nutrients and gas metabolism through a vascular circulation adjusted to the periods of development. This is an important component in achieving the growth and development of the human conceptus. An important morphological index in the assessment of placental status, reflecting the activity of metabolic processes, is the vascularization of chorionic villi [11]. Shchegolev et al. mention the importance of assessing the vascular component in the disturbance of vasculature in progressively stagnant pregnancies [11]. Placental vascularization can be assessed by evaluating angioendothelial components expressing the CD31 marker [5, 12].
CD31 represents the platelet endothelial cell adhesion molecule (PECAM-1), a highly glycosylated protein used in immunohistochemistry for endothelial cell determination, assessing the degree of angiogenesis. PECAM-1 is involved in angiogenesis through cell-cell adhesion with the formation of vascular tubules [13].
In this respect, the knowledge of the vascularization features and the evolving dynamics of the primary vascular network are important in addressing the impact of vascular perturbations in the chorio-villous germline status in pregnancies failure.
The aim of the current study was to assess vascular density by using anti-CD31 antibody in the chorio-villous germinal site in small term deregulated pregnancies.
Material and methods
The study was conducted at the Perinatal Center Level III, Institute of Mother and Child, during 2020. Tissue samples were obtained by uterine aspirate of 184 patients with term (3-12 w.g.) pregnancies clinically and morpho-pathologically diagnosed as evolving stagnant pregnancies. They were included in the study group, with evolving stagnant pregnancies (L1 = 144 cases), and those that evolved into early miscarriage (L2 = 40 cases) served as the study material. Patient ages ranged from 18-46 years (30.03±6.43) for group I and 17-42 years (30.85±6.36) for group II. All patients underwent ultrasonographic investigation (USG) to determine the gestational term after the first day of the last menstrual bleeding, as well as clinical diagnosis of early miscarriage (EM) or evolving stagnant pregnancy (SP). Tissue from the germinal sac of pregnancies terminated for social indications was used as a control group. This tissue was from 18 patients aged 22-40 years (30.5±5.6) and divided into two subgroups: a) 6-9 w.g. (n = 9 cases) and b) 10-12 w.g. (n = 9 cases).
The clinical data were obtained from patients' medical records based on their informed consent. The current research is part of a larger study of early-term dysregulated pregnancies within the state program "Morphological approach by conventional, histo- and immunohistochemical methods of the features of the pathological profile of early placentogenesis in early-term dysregulated pregnancies", number 20.80009.8007.17 P1P2 0750. Cases were selected in accordance with the inclusion and exclusion criteria:
Inclusion criteria: pregnancies terminated with gestational term from 3 to 12 w.g. (clinically confirmed, by USG and resolved in IMC); pregnancies with pathological evolution: stagnant, early miscarriage; pregnancies with abortion at social indications; quality and volume of the aspirate: chorionic villi and decidual plates in sufficient volume to form the standard paraffin block (1.0x1.0x0.5cm); monofetal pregnancies; lack of an age threshold.
Exclusion criteria: severe somatic pathology; multiple pregnancies; pregnancies terminated on medical indication; lack of clinical-anamnestic data in clinical examination records; lack of gestational term specification and USG confirmation of pregnancy status.
The technical procedure included histoprocessing of tissue samples, application of the usual histological method (haematoxylin-eosin), immunohistochemical method (anti-CD31) with evaluation of histopathological features and immunoexpression, and statistical processing.
The primary processing. Conceptus tissue was harvested at short term in obstetric units with rapid fixation in a 10% formalin solution, pH 7.2-7.4, to reduce the risk of early lysis of tissue material and bacterial flora overgrowth. The fixation period in a 10% buffered formalin solution lasted 24 hours. For paraffin embedding, the DP500/CIT2002 (Bio-Optica, Italy) system has been used. For histochemical and histological processing of samples, the "TISSUE-TEK, VIP 6AI" (Sakura, Japan) histoprocessor has been utilized. The 3.5-µm thick sections were obtained using the HM325 microtome (Thermoscientific) (USA) and placed on positively charged slides (APTACA, Italy) to be dyed.
Histological method. The sections were stained by the conventional classical hematoxylin-eosin (H.E.) method using Mayer hematoxylin (HEMM-36/21, BIOGNOST, Slovenia) and 1% eosin Y (EOY10-35/21, BIOGNOST, Slovenia). The H.E. sections were automatically stained with the AUS-240 autostainer, (Bio-Optica, Italy) and automatically mounted (TISSUE-TEK, ClasTM, Sakura, Japan). Suitable sections (with sufficient tissue material) were selected for immunohistochemical staining.
Immunohistochemical method. Immunohistochemical assays were performed using manually adapted operational procedures for anti-CD31 antibody (clone JC70A) with the application of the EnVisionTMFLEX detection system, high pH (K8000) [14]. The conventional immunohistochemical method was applied (Table 1). Deparaffinization was performed in two toluene baths (code UN1294, Sigma-Oldrich), the first bath for 60 min at 59°C in a thermostat, followed by the second bath for 5 min at room temperature, a mixed bath of toluene and 96% alcohol for 5 min, then 2 baths of 96% alcohol with re-hydration in 2 taps of 10 min each in distilled water. For the epitope demarcation purpose, the sections planned for anti-CD31 antibody disclosure were exposed to dissolved Target Flex solution (1ml Target: 49 ml distilled water) at high pH, during 20 minutes at 95ºC-96ºC with a total pretreatment and posttreatment time of 60 minutes. Incubation with anti-CD31 was carried out for 20 minutes at room temperature. After incubation with the primary antibody, neutralization of endogenous peroxidase with peroxidase block was performed for 5 minutes, followed by the application of the secondary antibody (HRP) for 20 minutes and DAB (3,3'-diaminobenzidine) applied as a chromogenic substrate for 5 minutes. Nuclei were counterstained with Mayer hematoxylin (HEMM-36/21, BIOGNOST, Slovenia). CD31-positive reaction was manifested in endotheliocytes with brown staining of the cell membrane. Then, the histological slide panel was subjected to the dehydration and clarifying procedure by two absolute drops of alcohol, one mixed drop of alcohol and toluene and three drops of toluene, each exposure lasting 5 minutes. The final procedure consisted of mounting the slides with BMC-100 solution. In the manual immunohistochemical staining procedure, Sequenza TM Immunostaining Center was applied using Thermo Shandon Coverplate.
Immunohistochemical testing with anti-CD31 was performed on a numerically smaller batch selected after evaluation (n = 80) and divided into: L1 = 43 cases; L2 = 12 cases; L3 = 17 cases. The difference in cases was due to the loss of histological sections during the testing procedure, paraffin block completion, or lack of primary material.
Table 1. Antibody used: source, dilution, demasking system, detection system, incubation time. | |||
Antibody/clone | Source/incubation time/dilution | Retrieval system/time | Detection/time |
CD31/JC70A | 20 min, ready- to-use | Solution Target Flex, high pH /Water bath at temperature of 95°C-96°C/20 min | EnVisionTMFLEX, high pH |
Microscopic evaluation. Expression of CD31 (endothelial cell adhesion receptor) protein was detected at the membrane level, expressed by the presence of brownish color in the evaluated tissue. In all sections, blood vessels were quantified by the hot-spot method. Initially, the highest density of chorionic villi areas was identified at ×100 magnification. Subsequently, chorionic villi with vessels (VC v+) and those without blood vessels (VC v-) were counted in 3 visual fields in the areas with the highest chorio-villous density at ×100 magnification, calculating the placental vascularization index (PVI, %). This presented the ratio of the number of vascularized chorionic villi vs the total number of chorionic villi ×100. The following score was assigned:
negative – in the case of PVI ranging from 0-25%;
+1 – in the case of PVI ranging from 26-50%;
+2 – in the case of PVI ranging from 51-75%;
+3 – in the case of PVI ranging from 76-100%.
Immunoexpression intensity was assessed as 0 (absent); + (positive).
Next, in 3 visual fields within the highest chorio-villous density areas, at x400 magnification, each cell, group of cells, cell cords, immunopositive vessels vs chorio-villous stroma area (mcm2) were counted. Based on the presence/absence of lumen, they were classified as vessels with lumen (V/L+) and without lumen (V/L-). After that, chorio-vascularization indices (IV/L+ and IV/L-) were calculated according to the following formulas: (V/L+)/(V/T) x100 and (V/L-)/(V/T) x100, where (V/Total) - total number of cells, cell cords, immunopositive vessels which expresses vascular density. The above-mentioned structures were counted in each of the 3 study groups (EM, ASI, SP), grouped according to gestational term into the following groups: 3-5 w.g., 6-9 w.g. and 10-12 w.g. Microscopic evaluation was performed using the Axio Imager A2 microscope (Carl Zeiss, Germany) equipped with the AXIOCam MRc5 recording camera.
Data analysis. The results of the study were stored in an Excel 2007 database (Microsoft Office 2007) and were analyzed using SPSS software (SPSS Statistics 23.0, IBM, Chicago, IL, USA). The median, arithmetic average, and standard deviation were determined. The difference between groups of variables was tested by applying the t-student test. Results were considered statistically significant at p ≤ 0.05.
Results
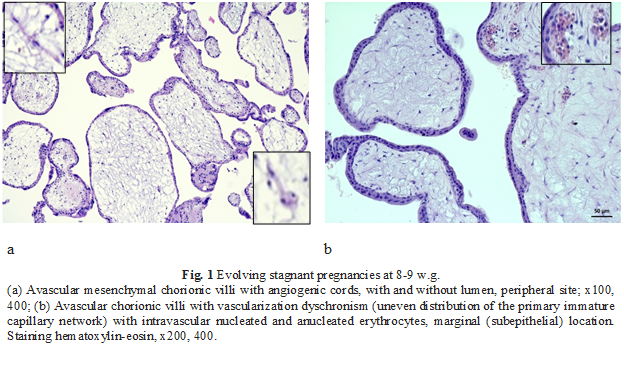
To assess the histomorphological vascularization features of the chorionic villi in the study groups, hematoxylin and eosin staining was initially applied. The majority of cases, 78.2% (n = 144), were presented by stagnant uterine pregnancies. Uterine pregnancies evolving into miscarriages accounted for 21.8% (n = 40). Vascularization disturbances were attested in the chorio-villous compartment and were manifested by vascular dyschronism: the presence of vascular and avascular chorionic villi in 100% of cases in all study groups, with a significant increase in the density of avascular chorionic villi in stagnant pregnancies (75%), Fig. 1.
To determine the percentage value of the placental vascularization index (PVI, %), the vascular density in the stroma of chorionic villi was assessed with the application of antibody with high specificity toward anti-CD31 endothelial cells. According to membrane immunostaining in the stroma of chorionic villi the following results were obtained, the numerical values and distribution of which are elucidated in Table 1.
According to the obtained results, anti-CD31 immunoexpression in the chorionic villus stroma induced PVI with a maximum mean in the control group (91.51±0.71) vs the EM and SP groups (82.29±12.96 and 57.47±6.53, respectively) (Table 2).
Table 2. Descriptive statistical analysis of placental vascular density: mean values and standard error of mean (±). | |||||||||
Vascular profile | Lot SA/D (M±SD), w.g. | Lot EM (M±SD), w.g. | Lot SP (M±SD), w.g. | ||||||
3-5 | 6-9 | 10-12 | 3-5 | 6-9 | 10-12 | 3-5 | 6-9 | 10-12 | |
VCtotal | - | 209.75±44.06 | 190.11 ±18.82 | 177.00 ±151.32 | 94.29 ±42.24 | 75.33 ±17.61 | 52.00 ±32.75 | 90.12 ± 40.98 | 77.64 ±22.15 |
VCv+ | - | 192.88±43.33 | 172.78 ±25.72 | 169.00 ±140.01 | 68.29 ±28.39 | 59.0 ±24.06 | 29,00 ±23.2 | 47.12 ±32.20 | 43,36 ±29.91 |
VCv- | - | 18.38 ±14.41 | 17.33 ±21.29 | 8.00 ±0.31 | 26,00 ±18,18 | 16.33 ±10.79 | 23.00 ±32.64 | 43.08 ±32.69 | 34.27 ±28.48 |
PVI% | - | 92.01±6.7 | 91.01 ±10.53 | 97.18 ±3.98 | 73.39 ±12.97 | 76.31 ±17.22 | 64.27 ±35.43 | 51.25 ±26.96 | 56.89 ±32.31 |
PVI% total | - | 91.51±0.71 | 82.29±12.6 | 57.47±6.53 | |||||
Note: M - average; SD - standard deviation; w.g. - weeks of gestation; ASI - abortion at social indications; EM - early miscarriage; SP - stagnant pregnancies; VCtotal - chorio-villous density; VCv+ - chorionic villus with vessel; VCv- - chorionic villus without vessel; PVI - placental vascularity index. | |||||||||
In the group of pregnancies resolved at a social indication/desire, there was an indirect correlation between the total number of chorionic villi and gestational term (rs = -0.511, p = 0.036). Gestational term similarly correlated with the total number of vessels (rs = 0.702, p = 0.002) as well as with the number of vessels with lumen (rs = 0.746, p = 0.001).
In the miscarriage group, the total number of vessels was directly dependent on the gestational term (rs = 0.746, p = 0.001). In the group of stagnant pregnancies, no statistical correlations were determined for any of the analyzed parameters.
Examining overall cases, we identified a range of statistically significant correlations between gestational term, study group, total number of chorionic villi, villi with vessels, villi without vessels, total number of vessels, vessels with lumen, and vessels without lumen. These correlations are shown in Table 3.
Table 3. Statistically significant correlations between study group, gestational term and various indices representing the vascular density. | ||||||||||
| TG | Lot | CV total | CVv+ | CVv- | Surface | V/Total | V/L+ | V/L- | |
TG | rs |
| -.245* | .209 | .187 | -.057 | .237 | .345 | .237 | .235 |
p |
| .038 | .077 | .115 | .633 | .046 | .003 | .047 | .049 | |
Lot | rs | -.245* |
| -.620 | -.740 | .313 | .390 | -.480 | -.554 | -.175 |
p | .038 |
| <.01 | <.01 | .007 | .0007 | .00002 | <.01 | .14372 | |
Note: rs - Spearman's correlation coefficient; GT - gestational term; CVtotal - chorio-villous density; CVv+ - chorio-villous villi with vessel; CVv- - chorio-villous villi without vessel; V/Total - chorio-villous vascular density; V/L+ vessel with lumen; V/L- vessel without lumen; * - statistically significant results. | ||||||||||
Further, the placental vascular density as a whole was analyzed, with a score given for PVI in the groups studied. The assignment of cases according to the score distribution is shown in Table 4.
In relation to gestation term, IVP in the control group was dominant in the subgroup (6-9) with a distribution score of +3 in 100%, and in the subgroup (10-12), a maximum score was attested in 88.9% vs one case (11.2%) with a score of +2. Overall, the distribution score in the group of pregnancies resolved at social indications/desire shows a high placental vascularity index (+3) in 94.1%.
Table 4. Distribution of IVP anti-CD31 immunoreactivity (score). | |||||
Unit group/subgroup/GT n = 72 | Vessel/Stroma/score | ||||
0 | +1 | +2 | +3 | ||
SP (n = 43) | 3-5 | 2 | - | 1 | 4 |
6-9 | 5 | 7 | 7 | 6 | |
10-12 | 3 | 3 | - | 5 | |
EM (n = 12) | 3-5 | - | - | - | 2 |
6-9 | - | - | 4 | 3 | |
10-12 | - | - | 2 | 1 | |
ASI (n = 17) | 6-9 | - | - | - | 8 |
10-12 | - | - | 1 | 8 | |
Note: 0 = (0-25%); + = (26-50%); ++ = (51-75%); +++ = (76-100%). Immunoexpression intensity was assessed as: - (absent); + (present); GT - gestational term; SP – stagnant pregnancies; EM - early miscarriage; ASI -abortion at social indication. | |||||
The EM group was characterized by an equalization of the distribution score per total between subgroups, with +2 and +3 each accounting for 50%, and the subgroup (3-5) rated +3 in 16.7%. However, the maximum IVP distribution score was set for subgroup (6-9) at 58.3%. The SEP group was the most diverse in relation to the IVP distribution score. Thus, overall, the +3 score predominated (34.9%), the 0 and +1 scores each (23.25%), and the +2 score was at (18.6%), with the highest diversity in the corresponding subgroup (6-9 w.g. ) with an average distribution value between the (0-1) vs (+2+3) score, respectively 12/13 cases.
Subsequently, the median was determined, which served as a reference value to elucidate the degree of vascularization of the chorionic villi (low for values lower than the median and high for values higher than the median) (Table 5).
Table 5. Descriptive statistical analysis of placental and chorio-villous vascular density, S/T and degree of vascularity: mean values and standard error of mean ± | |||||
Unit group/subgroup/TG n = 72 | Vessel/Stroma/score | ||||
PVI (%) | S/T, µm2 | V/Total mean values, m | Degree of vascularization | ||
SP (n = 43) | 3-5 | 64.27±35.43 | 36336.63±22207.69 | 5±1.88 m = 4.6 | high |
6-9 | 51.25±26.96 | 40448.05±37666.43 | 5.23±2.73 m = 4.33 | high | |
10-12 | 56.89±32.31 | 53025.42±49273.26 | 6.89±5.51 m = 4.86 | high | |
EM (n = 12) | 3-5 | 97.18±3.98 | 12881.76±1021.90 | 7.23±0.44 m = 7.23 | high |
6-9 | 73.39±12.97 | 13069.66±6215.77 | 7.26±3.11 m = 8.47 | low | |
10-12 | 76.31±17.22 | 30450.56±5780.98 | 16.28±1.93 m = 15.33 | high | |
ASI (n = 17) | 6-9 | 92.01±6.7 | 19935.37±4490.42 | 6.84±1.32 m = 6.825 | high |
10-12 | 91.01±10.53 | 26784.26±19753.27 | 14.13±10.24 m = 9.58 | high | |
Note:m – median; SP - stagnant pregnancies; EM - early miscarriage; ASI - abortion at social indication; PVI - placental vascularity index; S/T - chorio-villous stromal area; V/Total - chorio-villous vascular density. | |||||
In Table 5, we can see that practically in all cases, there was a pronounced degree of vascularization. Therefore, the next step was to assess the quality of vascularization by determining the ratio of vessels with lumen (functional) to those without lumen (non-functional). The vascularization index with lumen (VI/L+) and the vascularization index without lumen (VI/L-) were thus determined, and the data obtained are shown in Table 6.
Table 6. Descriptive statistical analysis of placental and choriovascular vascular density, VI/L+ and VI/L-: mean values and standard error of the mean ±. | |||||
Unit group/subgroup/TG n = 72 | Vessel/Stroma/score | ||||
IPV (%) | V/Total (%) | VI/L+ (%) | VI/L- (%) | ||
SP (n = 43) | 3-5 | 64.27±35.43 | 5±1.88 | 23.2 | 86.6 |
6-9 | 51.25±26.96 | 5.23±2.73 | 23.5 | 76.4 | |
10-12 | 56.89±32.31 | 6.89±5.51 | 32.2 | 67.7 | |
EM (n = 12) | 3-5 | 97.18±3.98 | 7.23±0.44 | 7.6 | 92.3 |
6-9 | 73.39±12.97 | 7.26±3.11 | 44.9 | 54.8 | |
10-12 | 76.31±17.22 | 16.28±1.93 | 21.3 | 74.8 | |
ASI (n = 17) | 6-9 | 92.01±6.7 | 6.84±1.32 | 53.6 | 46.9 |
10-12 | 91.01±10.53 | 14.13±10.24 | 52.3 | 50.2 | |
Note: TG - term of gestation; PVI - placental vascularity index; V/Total - chorio-villous vascular density; VI/L+ - vascular index with lumen, VI/L- - vascular index without lumen; SP - stagnant pregnancies; EM - early miscarriage; ASI - abortion at social indication. | |||||
In Table 6, we can see that the largest discrepancy between luminal and non-luminal vessels is found in the batch of stagnant pregnancies. Statistical analysis confirmed this observation. Thus, in this group, the difference between the density of vessels with lumen and those without lumen was statistically true (p = 0.011).
The hypothesis that each batch, depending on gestational term, was associated with statistically significant different mean values of PVI and blood vessels with lumen or without lumen was subsequently examined. The hypothesis was further investigated by applying the t-student test. The results of the intergroup differences in average means are reflected in Table 7.
Table 7. The difference in average values of IVP and lumen vessels, t-student test. | ||||
Weeks of gestation, w.g. | Lot | Results for PVI | Results for vessels with lumen | Results for vessels without lumen |
3-5 | EM vs SS | t6.467 = 2.405* p = 0.05 | t7 = -0.563 p = 0.591 | t7 = 2.289 p = 0.056 |
6-9 | AS/D vs AS | t8.720 = 3.421* p = 0.008 | t7.182 = 0.457 p = 0.661 | t13 = -1.296 p = 0.218 |
AS/D vs SS | t30.287 = 6.92* p<0.01 | t31 = 5.500* p = 0.000005 | t31 = -0.952 p = 0.348 | |
EM vs SS | t21.454 = 3.03* p = 0.006 | t6.905 = 2.266 p = 0.058 | t30 = -0.02 p = 0.984 | |
10-12 | AS/D vs AS | t10 = 1.812 p = 0.1 | t10 = 0.91 p = 0.384 | t10 = -2.067 p = 0.066 |
AS/D vs SS | t12.502 = -3.294* p = 0.006 | t10.688 = -2.069 p = 0.064 | t18 = 1.679 p = 0.11 | |
EM vs SPP | t12 = -0.983 p = 0.345 | t12 = 0.588 p = 0.567 | t12 = 3.862* p = 0.002 | |
Note:w.g. - weeks of gestation; ASI - abortion at social indication; EM - early miscarriage; SP - stagnant pregnancies; PVI - placental vascularization index; *- statistically significant results. | ||||
As shown in Table 7, statistically significant differences were established for PVI in most groups except for spontaneous abortion vs stagnant pregnancies at 10-12 w.g. Concurrently, there were statistically significant differences between the means of vessels with lumen in SA/D and SP (t31 = 5.500, p = 0.000005). The means of vessels without lumen showed statistically significant differences in spontaneous abortion and stagnant pregnancies groups (t12 = 3.862, p = 0.002).
Discussion
The assessment of fetal conceptus (FC) pathology in the early term remains a major challenge for obstetricians, sonographers, geneticists, and, not least, pediatric morphologists, as an integral part of clinical-morphological diagnostic management. According to the literature, as well as our own data, along with the increase in the number of abortions for social/medical indications, the incidence of stagnant pregnancies in evolution or those evolving into early miscarriages is reported to be quite high, ranging from 10% to 51% [15, 16].
Among the causes of miscarriages or stagnation of pregnancies are maternal-fetal factors and various multifactorial pathogenetic mechanisms that induce disorders of conceptus morphogenesis at the blastocyst and gastrulation stage, because of which various structural and phenotypic abnormalities develop [17]. Furthermore, for the formation of a long-term stable connection of the embryo/fetus placental feeding, it is necessary to initiate autochthonous vascularization within the chorio-villous mesenchymal stroma and to install the chorio-allantoic circulation in the formation of the embryo-hemochorionic circulation with the occurrence of the chorionic plate and chorionic villi vascularization [18].
In this regard, the placenta is an important connecting organ within the mother-placenta-fetus system, carrying out the transport of nutrients, conducting gaseous metabolism through a vascular circulation adjusted to the periods of development, serving as an important link in achieving growth and development of the human conceptus. An essential morphological index in placenta condition assessment, revealing the activity of metabolic processes, is the vascularization of chorionic villi [11]. Similarly, to our results, Shchegolev et al. mentioned the importance of assessing the vascular component in the disruption of blood supply in progressively stagnant pregnancies with a harmful effect on pregnancy [11].
The placental vasculature can be evaluated by assessing angio-endothelial components expressing the CD31 marker [5, 12], where CD31 represents platelet endothelial cell adhesion molecule (PECAM-1), a highly glycosylated protein used in immunohistochemistry to determine endothelial cells, assessing the degree of angiogenesis. Similarly, PECAM-1 is involved in angiogenesis through cell-cell adhesion with vascular tube formation [13].
In the current study, a pronounced disruption of villous chorion vasculature was established in evolving stagnant pregnancies with pronounced vascularity in most cases. Upon analysis of CD31 marker expression in endothelial cells in relation to the study groups, a discrepancy was determined at the vascularization stage with the involvement of vasculogenesis and angiogenesis with the dominance of vessels without lumen in the (SP) group, which in this case represents vascular retardation. According to Pereteatko L.P. et al., the comparative analysis of pregnancies in abortive disease and chronic endometritis denotes the delayed impact of both chorionic villi and vasculo-angiogenesis [3]. At the same time, disruption of growth factor content together with the accentuation of inflammatory factors’ effect in the given case contributes to the initiation of vascularization, the latter having a particular priority in the establishment of embryo-placental circulation [9, 19].
According to published data, vasculogenesis and angiogenesis during intrauterine development are important steps in establishing the embryo-feto-placental circulation and by the 3rd week, virtually all chorionic villi are secularized [20]. Another important milestone is the initiation of vasculogenesis in the yolk sac wall and embryo tissue, then in the mesenchyme of the chorionic plate and stroma of the secondary chorionic villi, with the fusion and formation of an integral embryo-hemochorionic blood circulation beginning around the 7th week. Any dysregulation of the formative stage of this system contributes to intrauterine death [21].
Conclusions
The degree of chorionic villi vascularization is different depending both on the mode of resolved pregnancy and gestational term, with statistically significant differences in PVI.
The evolutionary success of pregnancy is dependent not only on the degree of vascularization, but also on the quality of the vascular network, which can undergo changes through retardation or immature compensation (vessels with lumen and/or without lumen).
Disruption of placental vasculature is one of the basic links in the development of primary placental insufficiency during placentation.
Competing interests
None declared.
Authors’ contribution
VD designed the study, conducted the laboratory work, interpreted the data, and drafted the first manuscript; VF collected the material, interpreted the data; LS collected the material, interpreted the data; EC performed the laboratory work, interpreted the data; EF collected the material, interpreted the data; VF conducted the laboratory work, collected the material, interpreted the data; LS revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Ethical statement and patient consent
No approval was required for this study.
Funding
This work was carried out within the framework of the state project no. 20.80009.8007.17 „Morphological approach by conventional, histo- and immunohistochemical methods of the peculiarities of the pathological profile of early placentogenesis in short-term dysregulated pregnancies”.
Authors’ ORCID IDs
Valeriu David – https://orcid.org/0000-0001-9799-7369
Vergil Petrovici – https://orcid.org/0000-0001-8352-4202
Lilia Sinițîna – https://orcid.org/0000-0001-9646-8860
Ecaterina Carpenco – https://orcid.org/0000-0003-1464-3149
Ecaterina Foca – https://orcid.org/0000-0001-7629-4875
Veaceslav Fulga – https://orcid.org/0000-0002-7589-7188
Lilian Șaptefrați – https://orcid.org/0000-0003-2779-718X
References
Fedorova MV, Kalashnikova EP. Platsenta i ee rol' pri beremennosti [Placenta and its role during pregnancy]. Moscow: Meditsina;1986. 253 p. Russian.
Ailamazian EK, Kulakova VI, Radzinskii VE, editors. Akusherstvo: natsional'noe rukovodstvo [Obstetrics: national guidelines]. Мoscow: Geotar-Media; 2009. p. 263-271. Russian.
Peretiatko LP, Gulieva ZS, Gerasimov AM, Kuznetsov RA, Fateeva NV. Morfologicheskie i funktsional'nye izmeneniia endometriia pri privychnom nevynashivanii beremennosti u patsientok s nedifferentsirovannoi displaziei soedenitel'noi tkani [Endometrial morphological and functional changes in recurrent miscarriage in patients with undifferentiated connective tissue dysplasia]. Rossiiskii Vestnik Akushera-Ginekologa. 2017;17(1):14-20. doi: 10.17116/rosakush201717114-20. Russian.
Savel'eva GM, Fedorova MV, Klimenko PA, Sichinava LG. Platsentarnaia nedostatochnost' [Placental insufficiency]. Moscow: Meditsina; 1991. 276 p. Russian.
Aleksandrovich NV. Dinamika vaskuliarizatsii vorsin platsenty cheloveka v techenii fiziologicheskoi beremennosti [Dynamics of vascularization of human placental villi during physiological pregnancy] [dissertation]. Moscow; 2013. Russian.
Lisman B, Hoff M, Boer K, Bleker O, Groningen K, Exalto N. The architecture of first trimester chorionic villous vascularization: a confocal laser scanning microscopical study. Hum Reprod. 2007;22(8):2254-2260. doi: 10.1093/humrep/dem143.
Oppenraaij R, Koning A, Lisman B, Boer K, Hoff M, Spek P, Steegers E, Exalto N. Vasculogenesis and angiogenesis in the first trimester human placenta: an innovative 3D study using an immersive virtual reality system. Placenta. 2009;30(3):220-222. doi: 10.1016/j.placenta.2008.12.014.
Peretiatko LP, Fateeva NV, Kuznetsov RA, Malyshkina AI. Vaskuliarizatsiia vorsin khoriona v pervom trimestre beremennosti pri fiziologicheskom techenii i privychnom nevynashivanii na fone khronicheskogo endometrita [Vascularization of chorion villi in the first trimester of gestation with physiological course and in recurrent miscarriage with underlying chronic endometritis]. Rossiiskii Mediko-Biologicheskii Vestnik imeni I. P. Pavlova. 2017;25(4):612-620. doi: 10.23888/PAVLOVJ20174612-620. Russian.
Milovanov AP, Erofeeva LM, Zolotukhina IA. Morfogenez platsenty cheloveka v I trimester beremennosti [Morphogenesis оf human placenta in the first trimester of gestation]. Morfologiia. 2011;139(2):72-76. Russian.
Benirrschke K, Burton GJ, Baergen RN. Pathology of the human placenta. 6th ed. Heidelberg: Springer; 2012. 941 p.
Shchegolev AI, Liapin VM, Tumanova UN, et al. Gistologicheskie izmeneniia platsenty i vaskuliarizatsiia ee vorsin pri rannei i pozdnei preeklampsii [Histological changes in the placenta and vascularization of its villi in early- and late-onset preeclampsia]. Arkhiv Patologii. 2016;(1):13-18. doi: 10.17116/patol201678113-18. Russian.
Kayisli UA, Cayli S, Seval Y, Tertemiz F, Huppertz B, Demir R. Spatial and temporal distribuition of Tie-1 and Tie-2 during very early development of the human placenta. Placenta. 2006;27(6-7):648-659. doi: 10.1016/j.placenta.2005.05.013.
DeLisser HM, Christofidou-Solomidou M, Strieter RM, Burdick MD, Robinson CS, Wexler RS, et al. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol. 1997;151(3):671-677.
David V, Șaptefrați L, Fulga V, Rudico L, Petrovici V, Doncilă O. Protocolul tehnicii IHC manuale cu utilizarea anticorpului CD31, clona JC70A, sistemul de vizualizare EnVisionTM FLEX [Manual IHC technique protocol using CD31 antibody, clone JC70A, EnVisionTM FLEX visualization system]. Innovator certificate no. 5551. Chișinău; 2017. Romanian.
Magnus MC, Wilcox AJ, Morken NH, Weinberg CR, Håberg SE. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. BMJ. 2019;364: l869. doi: 10.1136/bmj.l869.
David V, Petrovici V, Sinițîna L, Grecichina E, Carpenco E, Cotruță A. Evaluarea incidenței morbidității conceptului fetal în sarcină dereglată la termen mic [Evaluation of the incidence of fetal conceptus morbidity in short-term disordered pregnancy]. Buletin de Perinatologie. 2020;3(88):111. Romanian.
Wang X, Chen C, Wang L, Chen D, Guang W, French J. Conception, early pregnancy loss,and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79(3):577-84. doi: 10.1016/s0015-0282(02)04694-0.
Serov VN, Manukhin IB, Kuz'min VN. Tsitomegalovirusnaia infektsiia v patologii beremennosti i ploda [Cytomegalovirus infection in pregnancy and fetal pathologies]. Akusherstvo i Ginekologiia. 1997;(6):16-19. Russian.
Kuznetzova AV. Khronicheskii endometrit [Chronic endometritis]. Arkhiv Patologii. 2000;62(3):48-52. Russian.
Sokolov DI. Vaskulogenez i angiogenez v razvitii platsenty [Vasculogenesis and angiogenesis in development of a placenta]. Zhurnal Akusherstva iZhenskikh Boleznei. 2007;56(3):130-133. Russian.
Milovanov AP, Ozhiganova IN. Embriokhorial'naia nedostatochnost': anatomofiziologicheskie predposylki, obosnovanie, definitsii i patogeneticheskie mekhanizmy [Embryochorionic insufficiency: anatomic and physiologic prerequisites, rationale, definitions and pathogenetic mechanisms]. Arkhiv Patologii. 2014;(3):4-8. Russian.