Introduction
Tics are sudden, rapid, recurrent, arrhythmic movements and vocalizations in variable frequency, type, and intensity, which usually appear in bouts and are performed without any specific purpose [1]. Tics can be motor and vocal (phonic).
Gilles de la Tourette syndrome (GTS) is a combination of the multiple motor tics with at least one vocal, starting before the age of 18 and persisting more than 12 months [1]. The intensity of tics in GTS can widely vary from mild tics in some patients to the violent tics or even combination of tics of different intensities in other patients.
The prevalence of GTS, although previously underestimated, is relatively high. GTS is present in almost 0.3-1% of the population. GTS is found in about 1-2% of children and 0.3-0.5% of adults [1-4]. The prevalence of only chronic motor and of only vocal tics is of about 3-4% in the general population (adults and children) [5]. The transient tics are found in approximatively 20% of the pediatric population [6]. The tics are more common in children compared to the adults and more frequently affect boys than girls (ratio boys/girls of about 3-4:1) [1].
The exact prevalence of tics in the Republic of Moldova is not known yet, but the results of the screening study in children of the preschool age show that 2.05% of them had tics [7].
Tics represent a neurodevelopmental disorder with a neurologic background [1]. Although there are a lot of cases of primary tics without comorbidities, tics are often coexistent with some psychiatrically disorders as attention-deficit/hyperactivity disorder (ADHD) and obsessive-compulsive disorder (OCD) [1].
The right diagnosis of tics is the key element of their adequate management. The therapeutically approach to the tics should be adapted to their intensity, degree of the impact of the quality of life of the patient and to the presence or absence of the comorbidities. In some cases, not tics but comorbidities should be the major goal of the treatment.
The etiopathogenetic basis of tics includes changes in the basal ganglia and their connections (cortico-striato-thalamo-cortical circuits) during the development and maturation of the brain [8]. Tics are due to the dysfunction at the level of different neurotransmitters: dopamine, gamma-aminobutyric acid, glutamate, serotonin etc.
There is no treatment yet that would definitely eliminate tics forever. At the same time, the tics fluctuate spontaneously: they can increase or decrease or even disappear for some time, without any explicit cause. The majority of patients become tic-free at the age of 18-20. Therefore, if the tics are mild and do not affect significantly the quality of the patient’s life, they need no treatment. The tics are temporarily suppressed by the drugs that act on the receptors of the neurotransmitters implied in tics’ generation. However, these medications have some considerable side effects, and the decision to treat should be taken in each case individually.
Tics can be voluntarily suppressed for a short time. This property was used to develop particular behavioral strategies that facilitate this temporary suppression. GTS is not caused by psychoemotional reactions, but the already existent tics can be increased by stress, tiredness, and excitement [9]. Their intensity could be decreased by relaxation techniques.
Diagnosis and treatment of tics have been the subject of the in-depth research in the last years. The data of multiple studies have already confirmed some hypotheses and contradicted other ones. New drugs with fewer side effects were developed. However, the lack of some treatment options in RM significantly limits the possibilities of physicians and patients with tics. Therefore, an algorithm of diagnosis and treatment adjusted to the autochthonous conditions is required.
Material and methods
Initially the search by the key words „Tourette guidelines“ and „tic disorder guidelines“ was performed through the publications until October 2023 in the online database PubMed (US National Library of Medicine, National Institute of Health) [10]. After the examination of the titles, the author selected papers representing official guidelines for the diagnosis and treatment of the tic disorder and GTS.
Afterwards was performed the search by the key words „Tourette diagnosis“, „Tourette treatment“, „tic diagnosis“ or „tic treatment” in the PubMed database through the publications between 2021 – October 2023. The option „humans” was active in the search settings to exclude preclinical studies. The inferior timeline limit was adjusted to the time of publication of the 2nd variant of the European clinical guidelines for Tourette syndrome and other tic disorders [1, 11-13], a critical evaluation of the valorous sources published until 2021. These guidelines were created by a large international group of specialists in GTS and consist of four parts.
To specify some notions and facts, additional sources of information were consulted. The diagnostic and therapeutic recommendations were assessed from the point of view of their relevance and accessibility in the Republic of Moldova (RM). The availability of the drugs in RM was checked on the site of the Medicines Agency and Medical Devices of RM [14].
The final text includes a synthesis of recommendations, with an algorithm of diagnosis and treatment of tics adapted for RM.
Results
Processing of the information. In the PubMed database were identified Canadian, German and two (1st and 2nd versions) European guidelines for GTS and tics. The 2nd variant of the European guidelines consists of four parts published apart, one of which includes the presentation and diagnostic approach to the GTS [1], and the remaining three parts describe the treatment of tics (behavioral, pharmacotherapeutic and neurosurgical approach) [11-13].
The Canadian guidelines for GTS, published in 2012, consist of two parts: the non-pharmacological treatment and the pharmacological treatment of tics [15-16]. The German guideline, appeared in 2012 is outdated and no more recommended by the German Neurological Society and is not available anymore on its official website. It shortly describes the clinical and diagnostical particularities of tics, as well as the therapeutic non-pharmacological and pharmacological opportunities, existent at the moment of publication. There is also a practice guideline recommendation summary from international authors, published in 2019, that summarized treatment recommendations for tics and Tourette disorders [17].
Of the articles published between the 2021-2015, 764 corresponded to the search criteria. After the analysis of the titles, 436 articles were qualified as probably most relevant for the topic of the present work. The text of 397 of them could be integrally accessed. Of them, the final bibliography of the article includes only the most relevant sources in the number of 95. In some cases (e.g., to define notions referring GTS, to precise statistical data, therapeutic methods or characteristics of some medications) additional literature (including publications before 2021) or the internet-sites were consulted.
Discussion
Diagnosis of tics. Tics represent the clinical manifestation of the neurodevelopmental disorder and belong to the spectrum of disorders including transient tics, chronic tics, GTS, ADHD and OCD. The phenotype and intensity of tics may significantly vary from barely observable and rare toviolent and frequent.
The main diagnostic option for tics is the clinical observation. The typical presentation does not need any supplementary investigation. The diagnosis of tics is based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition, Text Revision [18], that specifies:
Gilles de la Tourette syndrome: (1) Both multiple motor and at least one vocal tics have been present during illness, although not necessarily concurrently; (2) The tics may wax and wane in frequency but have persisted for more than 1 year since the first tic onset; (3) The onset is before the age of 18; (4) The disturbance is not attributable to the effects of a substance (e.g., cocaine) or another medical condition (e.g., Huntington’s disease, post viral encephalitis).
Persistent (Chronic) Motor or Vocal Tic Disorder: (1) Single or multiple motor or vocal tics have been present during illness, but not both motor and vocal; (2) The tics may wax and wane in frequency but have persisted for more than 1 year since the first tic onset; (3) The onset is before the age of 18; (4) The disturbance is not attributable to the effects of a substance (e.g., cocaine) or another medical condition (e.g., Huntington’s disease, postviral encephalitis); (5) Criteria have never been met for GTS.
Provisional Tic Disorder: (1) Single or multiple motor and/or vocal tics; (2) The tics have been present for less than 1 year since the first tic onset; (3) The onset is before the age of 18; (4) The disturbance is not attributable to the physiological effects of a substance (e.g., cocaine) or another medical condition (e.g., Huntington’s disease, post viral encephalitis); (5) Criteria have never been met for GTS or persistent (chronic) motor or vocal tic disorder.
Unspecified tic disorder: tics that do not meet the full criteria for a tic disorder or for any of the disorders in the neurodevelopmental disorders (e.g., the ones that appeared after the age of 18, the ones persisting less than 4 weeks etc.).
Other specified tics: refers to the tics that do not meet the criteria for a tic disorder or any specific neurodevelopmental disorder.
The tics in DSM-5-TR are included in the section „Neurodevelopmental Disorders”, category „Motor Disorders”.
There are also criteria of diagnosis defined by the International Classification of Diseases, 11th edition (ICD-11) [19] that are similar to those of DSM-5-TR.
The diagnosis of tics is based on:
1. Anamnesis: The anamnesis is indispensable for the tics’ diagnosis, because it allows to establish their specific particularities. It is important to collect not only the anamnesis referring to the tics, but also to their common comorbidities (ADHD, OCD, impulse-control disorder) and to ask about the clinical signs of a neurological disorder with secondary tics. The antenatal and family history in children are indispensable for the differential diagnosis.
Therefore, during the discussion, the patient should be asked about [1, 20, 21]:
Tics’ particularities (which tics the patient has, where are they localized, how frequent and how strong are they);
Onset age (usually tics begin between the 5th and 8th years of life, mean age 5 years, although lower ages of onset reported in up to 40% of cases [1]), circumstances of the first manifestation;
Evolution of tics – diurnal fluctuations, decrease or absence during sleep, long-time fluctuations with possible vanishing for hours, days or even months, changes in the tics’ pattern, increase between 8-12 years of age, decrease after 18 year of age;
Short-time voluntary suppression of tics (not always possible in little children) and particular unpleasant feeling of an internal tension during the suppression;
Family history: although the transmission is non-mendelian, and tics are due to the combination of the genetic and ambient factors, the relatives of the patients can also present tics, ADHD or OCD.
Physical consequences of tics (pain, lesion etc.) and psychosocial impact (on the social relations, learning, work, sleep);
Evaluation of the psychosocial intrafamilial situation, financial state, interpersonal conflicts caused by tics;
Presence of the comorbidities: OCD (obsessions, rituals), ADHD (attention disorder, hyperactivity), depression, anxiety, impulse-control disorder;
Other pathological signs (including the neurological ones), other diseases in the anamnesis;
Treatment anamnesis, doses, and efficiency of the medication.
The diagnosis of GTS can be supported by the so-called Diagnostic Confidence Index [22].
2. Physical examination: The physical examination contributes to the right diagnosis. The general examination of the patient (symptoms of other somatic disorders or of a dysmorphism indicating a genetic syndrome) and an exhaustive neurological examination (detection of some secondary causes of tics) should be performed. The tics’ phenotype, their suggestibility (they increase when somebody talks to the patient about them) and the voluntary suppressibility for a short time are assessed. During the medical consultation, patients can consciously or unconsciously suppress their tics. In such a case, parents or relatives of the patient are asked to film the tics at home.
3. Paraclinical examination: Today there is no paraclinical examination indispensable for the diagnosis of typically manifested primary tics [1, 20]. The paraclinical exams are useful only if tics are atypical, e.g., first-time appearance of tics after the age of 18, presence of the symptoms suggesting another disease, antecedent of a cranial trauma, etc.
If the symptoms suggest a metabolic (e.g., Wilson disease) or an autoimmune (e.g., Sydenham chorea) condition, a blood analysis is performed (blood count, creatin kinase and ceruloplasmin level, glycemia), the antistreptolysin O titer is assessed, and the presence of the antiphospholipid or antineuronal antibodies is tested [20, 23]. If the additional symptoms suggest a metabolic, inflammatory or neurodegenerative disease, the urinalysis or cerebrospinal fluid analysis are indicated.
If the clinical presentation is more suggestive for an epileptic condition (convulsive or myoclonic seizures, absences etc.), electroencephalography should be performed. For the diagnosis of typical tics, without any additional suspect symptoms, the electroencephalography is useless [1, 23]. In some rare cases, electromyography could help to differentiate tics and myoclonus, although these two entities usually have different clinical appearance.
The magnetic resonance imaging scan is ordered in case of suspicion of traumatic, infectious, metabolic or neurodegenerative brain lesion. There are no specific structural changes on the magnetic resonance imaging scan of a patient with primary tics [23].
Genetic analysis is performed only for the scientific purpose. No consistent genetic changes common for a large population of patients with tics have been detected yet [1, 23-26].
The neuropsychological testing could discover comorbid ADHD, OCD or depression. If there is a suspicion of a coexistent autistic spectrum disorder, the patient should be evaluated by a specialist in this domain. The uncomplicated tic disorder and GTS do not induce any mental or physical abnormality. If there is a mental and/or physical handicap, another explanation of its origin should be searched [23].
As it has been already mentioned, the paraclinical examinations are useful only in some particular cases, when the clinical manifestation of the tics is atypical or there are other pathological signs. The unjustified and excessive use of different paraclinical methods is non-contributive for the diagnosis and treatment of tic disorder and GTS.
Differential diagnosis of the primary tics. The differential diagnosis of tics is a complex process that requires a sufficient knowledge of the clinical manifestation of neurological diseases similar to tics. In the majority of cases, the anamnesis and clinical examination can be sufficient for the right diagnosis of tics. It is important to take into account the individual variability and intensity of tics in GTS.
Primary tics and GTS should be differentiated from [1, 5, 26-31]:
Primary tics, associated with the major psychiatric comorbidities, such as autistic spectrum disorders, mental retardation, major ADHD, major OCD, etc. If comorbidities have a severe evolution while tics are mild, the treatment should be oriented against the symptoms most affecting patient’s quality of life. As an example, in a patient with major ADHD and extreme mild tics (e.g., blinking) only ADHD should be treated, even if the stimulant medications could somehow increase the tics.
Secondary tics, that could be:
Caused by drugs and other substances, e.g., amphetamines or other central nervous system stimulants, serotonin reuptake inhibitors, cocaine, levodopa, carbamazepine, phenytoin, phenobarbital, lamotrigine, caffeine and other dopamine receptor blockers (tourettism and tardive tics);
Caused by hereditary diseases, e.g., neuroacanthocytosis, tuberous sclerosis, Wilson’s disease, neuroferritinopathy, Lesch-Nyhan syndrome (purine metabolism disorder), phenylketonuria etc. Tics are not the only manifestation of these diseases, and usually there are other pathologic manifestations;
Caused by infectious or autoimmune diseases, e.g., Sydenham’s chorea, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS), antiphospholipid syndrome, viral encephalitis, neurosyphilis, and Lyme’s disease. Other infectious, inflammatory and central nervous system dysfunction signs are usually present;
Tics associated with chromosomal syndromes, e.g., fragile X syndrome, Down’s syndrome, Kleinfelter’s syndrome, XYY karyotype, X trisomy, partial trisomy 16, Beckwith-Wiedemann syndrome, etc. A somatic dysmorphism and other specific symptoms are usually observed;
Tics caused by brain traumatisms or even by peripheral traumatism (e.g., tics after shoulder injury);
Other causes of tics: vascular stroke with lesion in the fronto-subcortical circuit, hypoxic-ischemic encephalopathy, etc.
Other movement disorders similar with tics: stereotypies, habits, mannerisms, rituals, chorea, ballism, myoclonus, dystonia, tremor, seizures (especially the myoclonic ones), akathisia, hyperekplexia, synkinesis, compulsions, restless legs syndrome, psychiatric disorders with pathological movements, psychogenic movement disorders.
Tics are not psychogenic and should be differentiated from the pathological movements in somatoform disorders that should be treated differently. It could be difficult to differentiate tics from compulsions in OCD, especially if they are comorbid. In table 1 are presented some differential criteria of tics and compulsions.
| Table 1. Differences and similitudes between tics and compulsions in OCD* | |
Tics | Compulsions in OCD |
Differences | |
| Purposeless | Goal-oriented actions, with a purpose (e.g.: „If I would not flap my hands three times, I will not be lucky today”). |
| Accompanied by urge to tic | Accompanied by obsessive thoughts |
| Usually are not associated with anxiety | Usually are associated with anxiety |
| Semivoluntary or involuntary | Voluntary |
| Typical onset age of 6-8 | Typical onset age of more than 8 |
| Fluctuant natural evolution | No significant fluctuations in evolution |
Similitudes | |
| Suppressible for a short time | Suppressible for a short time |
| Decrease if the attention is focused on other things | Decrease if the attention is focused on other things |
| Increased by emotions | Increased by emotions |
Note: * The table is adapted from the European clinical guidelines for Tourette syndrome and other tic disorders-version 2.0. Part I: assessment [1]. | |
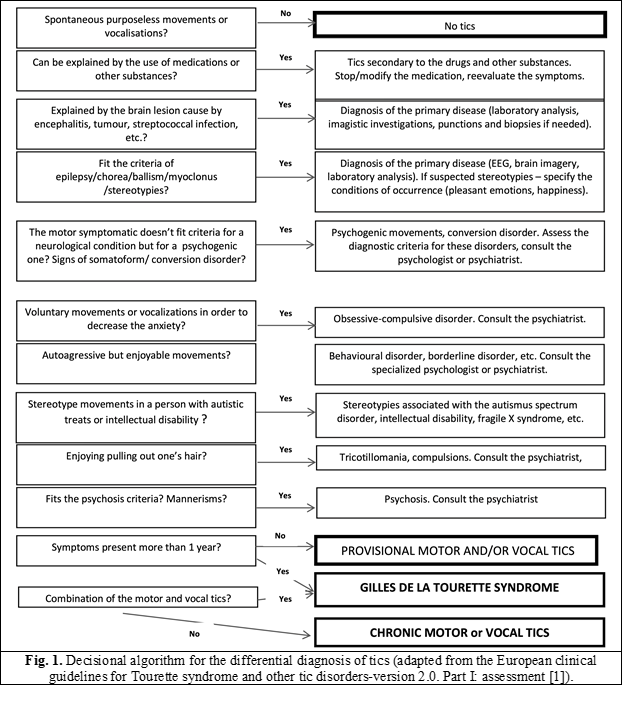
The diagnostic algorithm of tics is described in figure 1.
Treatment of tics. Selection of patients that need treatment. Only some patients with tics seek medical advice and only a part of them really need to be treated. The existent GTS medication does not provide total cure but just temporarily suppresses tics during the treatment. These drugs also have important adverse effects.
The modern scientific studies proved that tics have neural origin and represent a variant of the normal cerebral maturation. About 20% of all children have transient tics that will disappear spontaneously [6]. Other children have mild chronic not disturbing tics that do not affect their social life. The evolution of tic disorder and GTS is fluctuating and there could be weeks and even months free of tics. At the age of 18-20, the tics decrease or vanish in 90% of patients, although it is not possible to individually predict their evolution [1, 26].
Therefore, a decisive moment of the therapeutic management of tics is the individual decision to treat or not. Tics with a recent onset usually disappear in some weeks or months and do not need any treatment. In the majority of cases, the chronic tics will spontaneously disappear after some years, too. However, tics should be treated in the following conditions [1, 26, 27, 32]:
Tics are violent and cause muscular or neuropathic pain or even skin lesions (e.g., biting tics), muscular lesions, articular luxation, bone fractures.
Tics cause social problems, for example isolation and stigmatizing. As the parents frequently tend to overestimate the impact of tics, as well as adolescent patients, it is important to evaluate the real influence of tics and comorbidities on social life of the patient.
Tics induce major negative psychoemotional reactions as reactive depression, phobias, anxiety, low self-esteem, etc. In this case, psychological support and treatment should be also offered.
Tics interfere with the functional abilities of the patient. For example, the academic performance could decrease if a patient is all the time busy with tics’ suppression. Sometimes the motor or vocal tics could affect efficient communication with other people.
Management of patients needing no treatment. The only desire of the parents to treat their child with tics should not serve as indication to start the treatment if tics are mild and do not affect the psychoemotional, physical state and the social integration of the child. In such case, parents should be informed about the side effects of the tics’ medication that exceed the benefits for this patient. In the same way adult patients with mild tics should be informed. These people should be supervised and psychoeducated, and a psychological support should be offered if needed. In other words, for mild non-disturbing tics, the so-called „watch and wait” approach is preferred [1, 26, 27].
The psychoeducation includes [1, 17, 26, 27, 33-35]:
Information of the patient or parents in a clear and simple way about the clinical and etiopathogenetic particularities of tics. It is important to specify, that tics are not a malign disease, not a rare disorder, and represent a variant of a normal development;
Information about the fluctuant (waxing and waning) evolution and age-related particularities of the tics. The factors that could positively or negatively influence the tics’ intensity and frequency should be specified. The high probability that tics will disappear after the age of 18 should be accentuated;
Description of the typical comorbidities of the tics;
Explanation of the importance to inform the pre-school or school staff (for children with tics), the coworkers, and relatives. An adequate and clear information about the tics’ nature frequently ameliorates the social relationships.
Some people with mild tics could have severe comorbidities (major ADHD or OCD, impulse control disorder, depression, etc.), that need a specific treatment. As tics fluctuate in time, there could be periods when they increase and need a short-term treatment until the next „remission” when tics calm again.
Management of patients needing treatment: non-pharmacologic non-surgical methods. The already described psychoeducation is a key element in the management of any patient with tics. Recent studies proved that paying attention to tics contribute to their exacerbation, while they decrease when ignored. Therefore, some experts consider that the person with tics and his or her ambiance should try to ignore the tics, and that the voluntary suppression of tics decreases the probability that they will disappear [36].
At the same time there are many behavioral methods based on voluntary suppression of tics. They proved to be efficient in some patients. The disadvantage of behavioral treatment is the need for a permanent concentration on tics’ suppression, what is difficult for a person with an active everyday life. Additionally, the majority of patients feel a specific unpleasant sensation during the tics’ suppression, similar to a pressure, internal tension or itching – the so-called urge. Sometimes this feeling is unsupportable. There are also some persons with tics who have no premonitory sensation and, thus, are not eligible for behavioral treatment. Patients with few mild tics could especially benefit from such kind of therapies.
The two most capable behavioral methods are [11, 26, 37-43]:
The Habit Reversal Therapy – the patient is trained to observe the moment when a tic appears and to activate the antagonist muscles in order to stop the tic with a competitive movement;
The Exposure and Response Prevention – the patient is trained to suppress the tic, trying to habituate to the unpleasant sensations.
The behavioral therapy must be performed by a psychotherapist specially trained for this. Psychoanalysis or other methods used for the treatment of psychogenic movement disorders and conversion disorders are not recommended for GTS and tic disorders, because tics have a neural origin. At the same time, a psychological support could be used to cope with depression, stress, low self-esteem, and intrafamilial conflicts caused by tics.
Several novel treatment delivery formats of psychotherapy in tics are currently being evaluated, of which videoconference has the most evidence to date [11, 26, 39, 40, 44].
The anti-stress relaxation methods decrease the excitability of the neural structures connected with the neuronal source of tics. These methods have a benefic effect against the intensity and frequency of tics. Sport activities and, especially, swimming, have a positive effect on tics. Stress, anxiety, insufficient sleep, stimulant’s consumption (e.g., strong coffee and tea) increase tics [1, 11, 26].
Management of patients needing treatment: pharmacotherapy. The etiopathogenetic mechanism of tics is not yet known. Therefore, GTS and tic disorders are treated with the drugs acting on different neurotransmitter systems (e.g., dopaminergic, serotoninergic, noradrenergic, glutamatergic, GABA-ergic, cholinergic, opioid systems). During the evaluation of the efficiency of the drug, therapy should consider the natural fluctuant evolution of the tics. If the period of the spontaneous remission or, vice versa, of intensifying of tics by chance coincide with the use of medication, it could lead to false conclusions about its efficiency. As there is a large phenotypic variability, no medication has proven effective for all patients with GTS and chronic tic disorder [12].
The treatment should be started with low doses that will be gradually increased until the desired effect is obtained. Total elimination of tics is not always aimed, nor possible. The necessary doses of medications are usually much lower than the doses of the same drugs for other diseases. After some months of an efficient treatment the doses could be progressively decreased even to the point of discontinuation of the drug. It is important to take into account the fact that existent medications do not cure, but only suppress the tics while administrated [12, 15, 45-50].
For the last 40 years in tics’ treatment are used the post-synaptic dopaminergic D2 receptors’ blockers that are efficient in about 70% of cases [50]. This group of medication includes neuroleptics, typical ones (haloperidol, pimozide) and atypical (aripiprazole, risperidone, olanzapine, quetiapine, ziprasidone). Although neuroleptics are the most used and very efficient anti-tics drugs, they can cause severe side effects (extrapyramidal symptoms, hyperprolactinemia, abnormality of the cardiac repolarization) [12, 45, 50-52]. At the same time, without any clear explanation, neuroleptic-induced tardive dyskinesia is rare in GTS patients [53]. Atypical neuroleptics are preferentially recommended for the treatment of tic disorder and GTS, because they have less side effects than the typical ones [15, 23, 12, 44-50].
Aripiprazole, olanzapine, quetiapine, and risperidone are atypical neuroleptics accessible in the Republic of Moldova [14]. Of them, aripiprazole and risperidone seem to be the most efficient. The way of administration and the dosage of these drugs are presented in table 2.
The atypical neuroleptic aripiprazole is largely used in the worldwide practice, and has a good effect on tics in patients older than 6 years, with less side effects than other neuroleptics. Aripiprazole should be started with very low doses (1.25-2.5 mg/day) and increased by 1.25-2.5 mg every week or by 5 mg every 2 weeks, until the desired effect (lower intensity and frequency of tics) is observed. The usual maintenance doses are 3-5 mg/day; in case of severe tics, they could be progressively increased up to 10-15 mg/day. Aripiprazole has a good effect on tics, which could be seen in 70% of cases [12, 15, 45, 54-58].
Table 2. Medication recommended in international guidelines for the Gilles de la Tourette syndrome and tic disorder* | |||||||
Drug | Indication | Initial dose (mg/day) | Therapeutical dose (mg/day) | Frequent side effects | Paraclinical examinations at the start of the treatment and its maintenance | ||
| Alpha-adrenergic agonists | |||||||
| Clonidine | ADHD†/GTS‡ | 0.05 or 0.1 patch | 0.1-0.4 (divided in 1-4 doses) patch – 0.1-0.3 1.0-4.0 (in 1-2 doses) | Orthostatic hypotension, sedation, somnolence, xerostomia, headache | BP||, ECG¶ | ||
| Guanfacine | 0.5-1.0 | ||||||
| Atypical neuroleptics | |||||||
| Aripiprazole | GTS | 2.5 | 2.5-3.0 | Sedation, akathisia, ES**, headache, increased appetite (less than other neuroleptics), orthostatic hypotension | Blood count, BP, ECG, weight, transaminases, blood glucose | ||
| Olanzapine | GTS/OCD§ | 2.5-5.0 | 2.5-20.0 (once a day) | Sedation, increase in appetite, akathisia |
| ||
| Quetiapine | GTS | 25-50 | 100-600 (in 2 doses) | Sedation, increase in appetite, agitation, orthostatic hypotension, rarely – ES | Blood count, BP, ECG, weight, electrolytes, transaminases, prolactin, lipidic profile, blood glucose | ||
| Risperidone | GTS/OCD | 0.25 | 0.25-6.0 (in 2 doses) | Sedation, increase in appetite, orthostatic hypotension, depression and dysphoria, rarely – ES | |||
| Benzamides | |||||||
| Sulpiride | GTS/OCD | 50-100 (2 mg/kg) | 2-10 mg/kg (in 2-3 doses) | Sedation, dyssomnia, increased weight, hyperprolactinemia with amenorrhea and galactorrhea | Blood count, ECG, weight, transaminases, prolactin, electrolytes | ||
| Tiapride | GTS | 50-100 (2 mg/kg) | 2-10 mg/kg (in 2-3 doses) | ||||
Note: *Adapted from the Clinical European Guidelines for Tourette syndrome and tic disorders, part II [45] and the European clinical guidelines for Tourette syndrome and other tic disorders-version 2.0, part III [12]. With bold are marked the drugs approved in the Republic of Moldova. Typical neuroleptics are not included, because nowadays there are other medications with lesser or fewer side-effects. Abbreviations: † – attention deficit hyperactivity disorder; ‡ – Gilles de la Tourette syndrome; § – obsessive-compulsive disorder; || – blood pressure; ¶– electrocardiography; ** – extrapyramidal symptomatic. | |||||||
Less side effects, but also a lesser efficacy have the alpha-adrenergic agonists: clonidine and guanfacine. Only clonidine is available in RM [14]. There are no large studies investigating the role of clonidine in tics’ treatment, but the small trials concluded that it has a better effect on tics in patients with a comorbid ADHD and has a positive influence on the ADHD symptomatic [45, 58-60]. Clonidine seems to play a role in the sensorimotor gaiting [61]. This drug is used in doses of 0.0025-0.0055 mg/kg/day in tablets or as a patch 0.1-0.3 mg/day (the patch is not available in RM). Usually, it starts with 0.05 mg/day before the sleep. The dose is gradually increased. The maximal maintenance dose is 0.3-0.4 mg/day divided in 2-3 doses [12, 15, 45]. Side effects of clonidine are presented in table 2.
Benzamides (tiapride, sulpiride, amisulpride) represent another group of drugs used for tics’ therapy, acting on the D2 dopaminergic receptors of the ventral striatum and limbic system, as well as on the 5HT3 and 5HT4 serotonin receptors [12, 15, 45, 62-64]. For the amisulpride large studies are missing. Sulpiride has a proved efficiency against tics of 59% [63]. This drug is accessible in RM. The particularities of the sulpiride administration are presented in table 2.
Benzodiazepines, GABA-ergic modulators, have a long story of use in GTS. Although they are efficient in tics’ treatment (more efficient than clonidine, the positive result is present in about 71% of cases [59]), their use is limited by the development of dependence in some weeks and, particularly, tolerance (progressively higher doses with an increasing frequency of administration are needed) [64]. Benzodiazepines also have other major side effects, e.g., sedation, somnolence, short-memory disturbance, concentration disorder, confusion, ataxia, paradoxical insomnia, and anxiety). The withdrawal can lead to important reactions: anxiety, insomnia, phobias, psychotic reactions, tremor, headaches, dyskinesia, etc., therefore, the discontinuation of the treatment should be gradual. One of benzodiazepines used for tics’ therapy is clonazepam (initial doses of 0.125-0.5 mg/day and maintenance doses up to 6 mg/day). It could be sometimes used as short-term acute treatment (days-weeks) in case of violent tics [65].
Vesicular monoamine transporter type 2 (VMAT2) inhibitors (tetrabenazine, deutetrabenazine) deplete the presynaptic stock of dopamine and serotonin, and block the postsynaptic dopamine receptors [66-67]. VMAT2 are not available in RM. Tetrabenazine is largely used worldwide for tics’ therapy, being the first intention drug in the United States of America [12, 15, 68-71]. The benefic action of tetrabenazine on tics was proved in many studies, oscillating between 33% and 94% [69]. Deutetrabenazine is well tolerated according to the trial data [72]. Some studies suggest that it can improve tics in GTS [73], while other trials failed to show its benefits [71]. Because of its pharmacokinetic properties, deutetrabenazine can be given twice daily, thus improving compliance [74].
Ecopipam, a first in class, selective dopamine 1 receptor antagonist, reduced tics in some trials to a greater extent than placebo, without observable evidence of common antipsychotic-associated side effects [75, 76].
A small double blind, randomized, controlled crossover trial of cannabis in adults with GTS showed an improvement of tics with the Δ9-tetrahydrocannabinol (THC) 10% compared with placebo [77]. Cannabinoids and cannabis are not available as medicine in RM.
Injections of botulinum toxin could be a local therapeutic option against tics, although the quality for evidence is very low [78-80]. Their action is explained by a focal denervation with an influence on the sensory component of the tics-related brain circuits [78]. The efficacy of the botulinum toxin is variable. It could be used for the treatment of simple motor tics, especially at the level of the face and neck, the dosage being of 2500-3000 U [15]. The side effects are usually local and temporary, and include hypophonia, ptosis, temporary loss of muscular force etc.
Although a randomized controlled trial confirmed a high efficiency of valproic acid in GTS [81], there are no large-scale studies confirming it, and the side effects after the long-term therapy with this drug (teratogenicity, endocrine hormonal side effects, and hepatotoxicity) limit its use especially in women of childbearing age and in children [82]. Thus, valproate is not recommended anymore for the tics’ treatment.
The coexistence of comorbidities implies an adjuvant treatment of ADHD (e.g., clonidine, methylphenidate, atomoxetine) [83, 84] or OCD (e.g., sertraline, fluoxetine, fluvoxamine) [85, 86] in usual doses.
The European guidelines for GTS and other tic disorders recommend to initiate pharmacotherapy with dopamine blocking agents, preferably aripiprazole. Other agents that can be considered as first-line therapy include tiapride, risperidone, and especially in case of co-existing attention deficit hyperactivity disorder (ADHD), clonidine, and guanfacine [12].
Management of patients needing treatment: surgical methods. GTS is considered refractory to treatment if there is no response to 3 different drugs, including neuroleptics of both types – typical and atypical (but not „typical or atypical”), adequately dosed and administered for a sufficiently long period of time, without any significant amelioration of tics or with development of severe side effects imposing the discontinuation of the treatment [87-89]. If possible, at least 12 sessions of behavioral therapy should also be tried. At the same time, there is no generally established definition available for “treatment refractoriness” in GTS, and exact number of “treatment-refractory” patients is unknown [13]. If nothing helps, another therapeutic option could be deep brain stimulation (DBS) [13, 16]. Due to the possible major complications [90-94], this method is limited to severe tics. The selection of the candidates for DBS is made on rigorous criteria [13, 16, 47, 90-98]. DBS’ targets are represented by different regions of striatum (e.g., globus pallidus internus) and thalamus. At the moment the neurosurgical treatment of GTS is not available in RM.
Other methods of tics’ treatment. Acupuncture, meditation, massage, phytotherapy, and physiotherapeutic methods give contradictory results, and the mechanism of their action on tics is not clear yet [45, 12]. Relaxing (listening to the music [99], resting) or directing attention towards other things (e.g., computer games), one could have less tics than usually, and this kind of activities could be benefic if individually adjusted. The physical activity, especially swimming, decreases tics, but the etiopathogenesis of this effect is unclear yet [100-101]. Morning light therapy in one study showed some small but statistically significant decrease in tic severity [102].
In the last year there was found evidence for the median nerve stimulation in the reduction of tics’ frequency [103]. The transcranial magnetic stimulation (usually of the supplementary motor area) represents an experimental treatment method whose results are variable and not usually reproducible [104-107].
The algorithm of the therapeutic management of tics is presented in figure 2.

Conclusions
The right diagnosis of tics is the key element of their successful therapeutic management. The diagnosis of tics usually requires nothing more than detailed anamnesis and a thorough physical examination of the patient. Only if there is an atypical presentation of tics and another disease is suspected, other investigations could be necessary for a differential diagnosis, e.g., laboratory analysis, brain imaging, electroencephalography, etc. Secondary tics are accompanied by the clinical symptoms of the underlying disease.
In childhood, tics frequently disappear spontaneously in some weeks or months, and do not need treatment. Chronic tics could persist for years and usually decrease at the age of 18-20 years. Only tics with a negative impact on the physical state or affecting social life of the patients require treatment. Providing the psychological support and informing about the benign nature of tics and the probability of their spontaneous resolution, could be much more important than the pharmacotherapy. Social problems could be decreased by explicitly informing the patient’s ambience about the origin and particularities of tics.
GTS and tic disorder do not have any psychogenic cause, therefore, psychoanalysis is not useful to treat tics. The psychologist’s help is needed to treat the accompanying depression, low self-esteem, and psychosocial problems. The behavioral psychotherapies seem to be efficient in tics, however they need perseverance and could be difficult to practice in everyday life.
The pharmacological treatment of tics includes dopaminergic antagonists (especially atypical neuroleptics, e.g., aripiprazole, quetiapine, risperidone), alfa-adrenergic agonists (clonidine), benzamides (sulpiride, amisulpride), and tetrabenazine. Not all of these drugs are available in the Republic of Moldova. Clonazepam is not the first-intention medication for tics because of the rapid development of dependence and tolerance. However, it could be used as an acute treatment to abort the spontaneous increase of the violent tics during the natural tics’ fluctuations. The valproic acid (valproate) is less efficient as the aforementioned drugs and could cause important side effects if administered for a long period, especially on the developing brain of children, as well as teratogenicity when used in women of childbearing age.
The neurosurgical methods consist of the deep brain stimulation of the striatal structures and thalamus. They are indicated for severe tics resistant to all other treatments. Candidates are selected according to rigorous criteria. Deep brain stimulation for GTS is not available in RM yet.
The treatment should be adapted to tics’ fluctuations, and sometimes the dosage should be progressively decreased or the drug should be even stopped.
The studies exploring other medication methods (acupuncture, phytotherapy, massage, meditation, etc.) give contradictory results. The physical activity and swimming have a positive influence on tics. Transcranial magnetic stimulation seems to have only a short-term effect on tics. A new electrical wrist device reducing tics was recently elaborated, based on the electrical median nerve stimulation study.
Patients with tic disorder or GTS could have specific psychiatric comorbidities, such as ADHD and OCD. The treatment of these comorbidities could significantly ameliorate quality of life of the patient.
The recommendations referring to the diagnosis and treatment of tics in this paper correspond to the results of the recent scientific studies. The adequate management of tics and their comorbidities assure a good quality of life of the patients and their optimal social integration.
Competing interests
In 2013, Valeria Sajin received the scholarships for the research in Gilles de la Tourette syndrome’s domain from the European Society for the Study of Tourette Syndrome (COST Action BM0905) and from the European Federation of Neurological Societies.
Funding
No funding was received for this article.
Abbreviations
DSM-5 – Diagnostic and Statistical Manual, 5th edition; DBS – Deep Brain Stimulation; GABA – gamma-aminobutyric acid; RM – Republic of Moldova; GTS – Gilles de la Tourette syndrome; ADHD – attention deficit hyperactivity disorder; OCD – obsessive-compulsive disorder.
Author’s ORCID ID
Valeria Sajin – https://orcid.org/0009-0007-8054-4313
References
Szejko N, Robinson S, Hartmann A, Ganos C, Debes NM, Skov L, Haas M, Rizzo R, Stern J, Münchau A, Czernecki V, Dietrich A, Murphy TL, Martino D, Tarnok Z, Hedderly T, Müller-Vahl KR, Cath DC. European clinical guidelines for Tourette syndrome and other tic disorders-version 2.0. Part 1: assessment. Eur Child Adolesc Psychiatry. 2022 Mar;31(3):383-402. doi: 10.1007/s00787-021-01842-2.
Centers for Disease, Control, and Prevention. Prevalence of diagnosed Tourette syndrome in persons aged 6-17 years, United States, 2007. Morb Mortal Wkly Rep. 2009;58(21):581-5.
Robertson M. The prevalence and epidemiology of Gilles de la Tourette syndrome. Part 1: the epidemiological and prevalence studies. J Psychosom Res. 2008;65(5):461-72. doi: 10.1016/j.jpsychores.2008.03.006.
Scharf JM, Gauvin C, Alabiso J, Mathews C, Ben-Shlomo Y. Population prevalence of Tourette syndrome: a systematic review and meta-analysis. Mov Disord. 2015;30(2):221-8. doi: 10.1002/mds.26089.
Jankovic J, Kurlan R. Tourette syndrome: evolving concepts. Mov Disord. 2011;26(6):1149-56. doi: 10.1002/mds.23618.
Scahill L, Specht M, Page C. The prevalence of tic disorders and clinical characteristics in children. J Obsessive Compuls Relat Disord. 2014;3(4):394-400. doi: 10.1016/j.jocrd.2014.06.002.
Coțofan N, Sajin V, Odobescu S, Moldovanu I. Prevalence estimates of tics in pre-school children in the Republic of Moldova. In: 1st World Congress on Tourette syndrome and tic disorders, 24-25 June 2015, London, UK: Abstract book. Lausanne; 2015. p. 165-166. ISBN 978-2-88919-669-2.
Worbe Y, Marrakchi-Kacem L, Lecomte S, Valabregue R, Poupon F, Guevara P, et al. Altered structural connectivity of cortico-striato-pallido-thalamic networks in Gilles de la Tourette syndrome. Brain. 2015;138 (Pt2): 472-82. doi: 10.1093/brain/awu311.
Caurín B, Serrano M, Fernández-Alvarez E, Campistol J, Pérez-Dueñas B. Environmental circumstances influencing tic expression in children. Eur J Paediatr Neurol. 2014;18(2):157-62. doi: 10.1016/j.ejpn.2013.10.002.
PubMed [Internet]. Bethesda (MD): National Library of Medicine; 2023- [cited 2023 Oct 19]. Available from: http://www.ncbi.nlm.nih.gov/pubmed
Andrén P, Jakubovski E, Murphy TL, Woitecki K, Tarnok Z, Zimmerman-Brenner S, van de Griendt J, Debes NM, Viefhaus P, Robinson S, Roessner V, Ganos C, Szejko N, Müller-Vahl KR, Cath D, Hartmann A, Verdellen C. European clinical guidelines for Tourette syndrome and other tic disorders-version 2.0. Part 2: psychological interventions. Eur Child Adolesc Psychiatry. 2022 Mar;31(3):403-423. doi: 10.1007/s00787-021-01845-z.
Roessner V, Eichele H, Stern JS, Skov L, Rizzo R, Debes NM, Nagy P, Cavanna AE, Termine C, Ganos C, Münchau A, Szejko N, Cath D, Müller-Vahl KR, Verdellen C, Hartmann A, Rothenberger A, Hoekstra PJ, Plessen KJ. European clinical guidelines for Tourette syndrome and other tic disorders-version 2.0. Part 3: pharmacological treatment. Eur Child Adolesc Psychiatry. 2022 Mar;31(3):425-441. doi: 10.1007/s00787-021-01899-z.
Szejko N, Worbe Y, Hartmann A, Visser-Vandewalle V, Ackermans L, Ganos C, Porta M, Leentjens AFG, Mehrkens JH, Huys D, Baldermann JC, Kuhn J, Karachi C, Delorme C, Foltynie T, Cavanna AE, Cath D, Müller-Vahl K. European clinical guidelines for Tourette syndrome and other tic disorders-version 2.0. Part 4: deep brain stimulation. Eur Child Adolesc Psychiatry. 2022 Mar;31(3):443-461. doi: 10.1007/s00787-021-01881-9.
Medicines and Medical Devices Agency of the Republic of Moldova. Nomenclatorul de Stat al Medicamentelor [State nomenclature of medicines] [Internet]. Chisinau: AMDM; 2023-[cited 2023 Oct 19]. Romanian. Available from: https://amdm.gov.md/ro/page/nomenclatorul_de_stat_amed
Pringsheim T, Doja A, Gorman D, McKinlay D, Day L, Billinghurst L, Carroll A, Dion Y, Luscombe S, Steeves T, Sandor P. Canadian guidelines for the evidence-based treatment of tic disorders: pharmacotherapy. Can J Psychiatry. 2012;57(3):133-43. doi: 10.1177/070674371205700302.
Steeves T, McKinlay B, Gorman D, Billinghurst L, Day L, Carroll A, Dion Y, Doja A, Luscombe S, Sandor P, Pringsheim T. Canadian guidelines for the evidence-based treatment of tic disorders: behavioural therapy, deep brain stimulation, and transcranial magnetic stimulation. Can J Psychiatry. 2012;57(3):144-51. doi: 10.1177/070674371205700303.
Pringsheim T, Okun MS, Müller-Vahl K, Martino D, Jankovic J, Cavanna AE, Woods DW, Robinson M, Jarvie E, Roessner V, Oskoui M, Holler-Managan Y, Piacentini J. Practice guideline recommendations summary: treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurology. 2019 May 7;92(19):896-906. doi: 10.1212/WNL.0000000000007466.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5-TR. 5th ed. Washington: American Psychiatric Association Publishing; 2022. doi: 10.1176/appi.books.9780890425787.
World Health Organization. International statistical classification of diseases and related health problems: ICD-11. 11th ed. [Internet]. Geneva: WHO; 2019- [cited 2023 Oct 19]. Available from: https://icd.who.int/
Martino D, Mink J. Tic disorders. Continuum (Minneap Minn). 2013;19(5):1287-311. doi: 10.1212/01.CON.0000436157.31662.af.
Eapen V, Usherwood T. Assessing tics in children. BMJ. 2022 Mar 3;376:e069346. doi: 10.1136/bmj-2021-069346.
Robertson M, Banerjee S, Kurlan R, Cohen D, Leckman J, McMahon W, Pauls D, Sandor P, van de Wetering B. The Tourette syndrome diagnostic confidence index: development and clinical associations. Neurology. 1999;53(9):2108-12. doi: 10.1212/wnl.53.9.2108.
Cath DC, Hedderly T, Ludolph AG, Stern JS, Murphy T, Hartmann A, et al. European clinical guidelines for Tourette syndrome and other tic disorders. Part 1: assessment. Eur Child Adolesc Psychiatry. 2011;20(4):155-171. doi: 10.1007/s00787-011-0164-6.
Lin WD, Tsai FJ, Chou IC. Current understanding of the genetics of tourette syndrome. Biomed J. 2022 Apr;45(2):271-279. doi: 10.1016/j.bj.2022.01.008.
Qi Y, Zheng Y, Li Z, Liu Z, Xiong L. Genetic studies of tic disorders and Tourette syndrome. Methods Mol Biol. 2019;2011:547-571. doi: 10.1007/978-1-4939-9554-7_32.
Verdellen C, van de Griendt J, Hartmann A, Murphy T; ESSTS Guidelines Group. European clinical guidelines for Tourette syndrome and other tic disorders. Part 3: behavioural and psychosocial interventions. Eur Child Adolesc Psychiatry. 2011 Apr;20(4):197-207. doi: 10.1007/s00787-011-0167-3.
Ueda K, Black KJ. A Comprehensive review of tic disorders in children. J Clin Med. 2021 Jun 3;10(11):2479. doi: 10.3390/jcm10112479.
Mejia NI, Jankovic J. Secondary tics and tourettism. Braz J Psychiatry. 2005 Mar;27(1):11-7. doi: 10.1590/s1516-44462005000100006.
Stern JS. Tourette's syndrome and its borderland. Pract Neurol. 2018 Aug;18(4):262-270. doi: 10.1136/practneurol-2017-001755.
Szejko N, Müller-Vahl KR. Challenges in the diagnosis and assessment in patients with Tourette syndrome and comorbid obsessive-compulsive disorder. Neuropsychiatr Dis Treat. 2021 Apr 30;17:1253-1266. doi: 10.2147/NDT.S251499.
Cohen SC, Leckman JF, Bloch MH. Clinical assessment of Tourette syndrome and tic disorders. Neurosci Biobehav Rev. 2013 Jul;37(6):997-1007. doi: 10.1016/j.neubiorev.2012.11.013.
Rizzo R, Gulisano M. Treatment options for tic disorders. Expert Rev Neurother. 2020 Jan;20(1):55-63. doi: 10.1080/14737175.2020.1698950.
Nussey C, Pistrang N, Murphy T. How does psychoeducation help? A review of the effects of providing information about Tourette syndrome and attention-deficit/hyperactivity disorder. Child Care Health Dev. 2013 Sep;39(5):617-27. doi: 10.1111/cch.12039.
Jakubovski E, Müller-Vahl KR. Gilles de la Tourette-Syndrom: Klinik, Ursachen, Therapie [Gilles de la Tourette Syndrome: Symptoms, Causes and Therapy]. Psychother Psychosom Med Psychol. 2017 Jun;67(6):252-268. German. doi: 10.1055/s-0043-103269.
Robertson MM, Eapen V, Singer HS, Martino D, Scharf JM, Paschou P, Roessner V, Woods DW, Hariz M, Mathews CA, Črnčec R, Leckman JF. Gilles de la Tourette syndrome. Nat Rev Dis Primers. 2017 Feb 2;3:16097. doi: 10.1038/nrdp.2016.97.
Misirlisoy E, Brandt V, Ganos C, Tübing J, Münchau A, Haggard P. The relation between attention and tic generation in Tourette syndrome. Neuropsychology. 2015;29(4):658-65. doi: 10.1037/neu0000161.
Frey J, Malaty IA. Tourette syndrome treatment updates: a review and discussion of the current and upcoming literature. Curr Neurol Neurosci Rep. 2022 Feb;22(2):123-142. doi: 10.1007/s11910-022-01177-8.
Liang JH, Zhang SX, Chen YC, Tan KY, Zhang JS, Zhao Y, Kakaer A, Chen YJ. Role of psychotherapy strategy for the management of patients with Tourette syndrome - a Bayesian network meta-analysis. J Psychiatr Res. 2021 Nov;143:451-461. doi: 10.1016/j.jpsychires.2021.07.051.
Viefhaus P, Adam J, Goletz H, Woitecki K, Döpfner M. Implementation and evaluation of therapeutic online coaching using habit reversal training in children with Tourette's disorder - a pilot study. Front Psychol. 2021 Nov 23;12:780539. doi: 10.3389/fpsyg.2021.780539.
Hollis C, Hall CL, Jones R, Marston L, Novere ML, Hunter R, et al. Therapist-supported online remote behavioural intervention for tics in children and adolescents in England (ORBIT): a multicentre, parallel group, single-blind, randomised controlled trial. Lancet Psychiatry. 2021 Oct;8(10):871-882. doi: 10.1016/S2215-0366(21)00235-2.
Rizwan M, Shahid NUA, Naguit N, Jakkoju R, Laeeq S, Reghefaoui T, Zahoor H, Yook JH, Mohammed L. Efficacy of behavioural intervention, antipsychotics, and alpha agonists in the treatment of tics disorder in Tourette's syndrome. Cureus. 2022 Feb 21;14(2):e22449. doi: 10.7759/cureus.22449.
Woods DW, Himle MB, Stiede JT, Pitts BX. Behavioral interventions for children and adults with tic disorder. Annu Rev Clin Psychol. 2023 May 9;19:233-260. doi: 10.1146/annurev-clinpsy-080921-074307.
Kim KM, Bae E, Lee J, Park TW, Lim MH. A review of cognitive and behavioral interventions for tic disorder. Soa Chongsonyon Chongsin Uihak. 2021 Apr 1;32(2):51-62. doi: 10.5765/jkacap.200042.
Haas M, Jakubovski E, Kunert K, Fremer C, Buddensiek N, Häckl S, Lenz-Ziegenbein M, Musil R, Roessner V, Münchau A, Neuner I, Koch A, Müller-Vahl K. ONLINE-TICS: Internet-delivered behavioral treatment for patients with chronic tic disorders. J Clin Med. 2022 Jan 4;11(1):250. doi: 10.3390/jcm11010250.
Roessner V, Plessen KJ, Rothenberger A, Ludolph AG, Rizzo R, Skov L, Strand G, Stern JS, Termine C, Hoekstra PJ; ESSTS Guidelines Group. European clinical guidelines for Tourette syndrome and other tic disorders. Part 2: pharmacological treatment. Eur Child Adolesc Psychiatry. 2011 Apr;20(4):173-96. doi: 10.1007/s00787-011-0163-7.
Hollis C, Pennant M, Cuenca J, Glazebrook C, Kendall T, Whittington C, et al. Clinical effectiveness and patient perspectives of different treatment strategies for tics in children and adolescents with Tourette syndrome: a systematic review and qualitative analysis. Health Technol Assess. 2016 Jan;20(4):1-450, vii-viii. doi: 10.3310/hta20040.
Billnitzer A, Jankovic J. Current management of tics and Tourette syndrome: behavioral, pharmacologic, and surgical treatments. Neurotherapeutics. 2020 Oct;17(4):1681-1693. doi: 10.1007/s13311-020-00914-6.
Deeb W, Malaty IA, Mathews CA. Tourette disorder and other tic disorders. Handb Clin Neurol. 2019;165:123-153. doi: 10.1016/B978-0-444-64012-3.00008-3.
Set KK, Warner JN. Tourette syndrome in children: an update. Curr Probl Pediatr Adolesc Health Care. 2021;51(7):101032. doi: 10.1016/j.cppeds.2021.101032.
Roth J. The colorful spectrum of Tourette syndrome and its medical, surgical and behavioral therapies. Parkinsonism Relat Disord. 2018 Jan;46 Suppl 1:S75-S79. doi: 10.1016/j.parkreldis.2017.08.004.
Shapiro E, Shapiro AK, Fulop G, Hubbard M, Mandeli J, Nordlie J, Phillips RA. Controlled study of haloperidol, pimozide and placebo for the treatment of Gilles de la Tourette's syndrome. Arch Gen Psychiatry. 1989 Aug;46(8):722-30. doi: 10.1001/archpsyc.1989.01810080052006.
Pandey S, Dash D. Progress in pharmacological and surgical management of Tourette syndrome and other chronic tic disorders. Neurologist. 2019 May;24(3):93-108. doi: 10.1097/NRL.0000000000000218.
Müller-Vahl KR, Krueger D. Does Tourette syndrome prevent tardive dyskinesia? Mov Disord. 2011 Nov;26(13):2442-3. doi: 10.1002/mds.23894.
Masi G, Gagliano A, Siracusano R, Berloffa S, Calarese T, Ilardo G, Pfanner C, Magazù A, Cedro C. Aripiprazole in children with Tourette's disorder and co-morbid attention-deficit/hyperactivity disorder: a 12-week, open-label, preliminary study. J Child Adolesc Psychopharmacol. 2012 Apr;22(2):120-5. doi: 10.1089/cap.2011.0081.
Wenzel C, Kleimann A, Bokemeyer S, Müller-Vahl KR. Aripiprazole for the treatment of Tourette syndrome: a case series of 100 patients. J Clin Psychopharmacol. 2012 Aug;32(4):548-50. doi: 10.1097/JCP.0b013e31825ac2cb.
Yoo HK, Joung YS, Lee JS, Song DH, Lee YS, Kim JW, Kim BN, Cho SC. A multicenter, randomized, double-blind, placebo-controlled study of aripiprazole in children and adolescents with Tourette's disorder. J Clin Psychiatry. 2013 Aug;74(8):e772-80. doi: 10.4088/JCP.12m08189.
Cox JH, Cavanna AE. Aripiprazole for the treatment of Tourette syndrome. Expert Rev Neurother. 2021 Apr;21(4):381-391. doi: 10.1080/14737175.2021.1893693.
Farhat LC, Behling E, Landeros-Weisenberger A, Levine JLS, Macul Ferreira de Barros P, Wang Z, Bloch MH. Comparative efficacy, tolerability, and acceptability of pharmacological interventions for the treatment of children, adolescents, and young adults with Tourette's syndrome: a systematic review and network meta-analysis. Lancet Child Adolesc Health. 2023 Feb;7(2):112-126. doi: 10.1016/S2352-4642(22)00316-9.
Goetz C. Clonidine and clonazepam in Tourette syndrome. Adv Neurol. 1992;58:245-251.
Leckman JF, Hardin MT, Riddle MA, Stevenson J, Ort SI, Cohen DJ. Clonidine treatment of Gilles de la Tourette's syndrome. Arch Gen Psychiatry. 1991 Apr;48(4):324-8. doi: 10.1001/archpsyc.1991.01810280040006.
Eapen V, Ward P, Clarke R. Clonidine in Tourette syndrome and sensorimotor gating. Psychiatry Res. 2014 Feb 28;215(2):494-6. doi: 10.1016/j.psychres.2013.10.009.
Mogwitz S, Buse J, Ehrlich S, Roessner V. Clinical pharmacology of dopamine-modulating agents in Tourette's syndrome. Int Rev Neurobiol. 2013;112:281-349.
Robertson MM, Schnieden V, Lees AJ. Management of Gilles de la Tourette syndrome using sulpiride. Clin Neuropharmacol. 1990 Jun;13(3):229-35. doi: 10.1097/00002826-199006000-00005.
Janhsen K, Roser P, Hoffmann K. The problems of long-term treatment with benzodiazepines and related substances. Dtsch Arztebl Int. 2015 Jan 5;112(1-2):1-7. doi: 10.3238/arztebl.2015.0001.
Eddy C, Rickards H, Cavanna A. Treatment strategies for tics in Tourette syndrome. Ther Adv Neurol Disord. 2011;4(1):25-45. doi: 10.1177/1756285610390261.
Jankovic J. Dopamine depleters in the treatment of hyperkinetic movement disorders. Expert Opin Pharmacother. 2016 Dec;17(18):2461-2470. doi: 10.1080/14656566.2016.1258063.
Niemann N, Jankovic J. Real-world experience with VMAT2 inhibitors. Clin Neuropharmacol. 2019 Mar/Apr;42(2):37-41. doi: 10.1097/WNF.0000000000000326.
Porta M, Sassi M, Cavallazzi M, Fornari M, Brambilla A, Servello D. Tourette's syndrome and role of tetrabenazine: review and personal experience. Clin Drug Investig. 2008;28(7):443-59. doi: 10.2165/00044011-200828070-00006.
Chen JJ, Ondo WG, Dashtipour K, Swope DM. Tetrabenazine for the treatment of hyperkinetic movement disorders: a review of the literature. Clin Ther. 2012 Jul;34(7):1487-504. doi: 10.1016/j.clinthera.2012.06.010.
Jankovic J. Treatment of hyperkinetic movement disorders. Lancet Neurol. 2009 Sep;8(9):844-56. doi: 10.1016/S1474-4422(09)70183-8.
Coffey B, Jankovic J, Claassen DO, Jimenez-Shahed J, Gertz BJ, Garofalo EA, et al. Efficacy and safety of fixed-dose deutetrabenazine in children and adolescents for tics associated with Tourette syndrome: a randomized clinical trial. JAMA Netw Open. 2021 Oct 1;4(10):e2129397. doi: 10.1001/jamanetworkopen.2021.29397.
Jankovic J, Coffey B, Claassen DO, Jimenez-Shahed J, Gertz BJ, Garofalo EA, Stamler DA, Wieman M, Savola JM, Harary E, Alexander J, Barkay H, Gordon MF. Safety and efficacy of long-term deutetrabenazine use in children and adolescents with tics associated with Tourette syndrome: an open-label extension study. Mov Disord Clin Pract. 2023 Aug 24;10(9):1388-1398. doi: 10.1002/mdc3.13849.
Jankovic J, Jimenez-Shahed J, Budman C, Coffey B, Murphy T, Shprecher D, Stamler D. Deutetrabenazine in tics associated with Tourette syndrome. Tremor Other Hyperkinet Mov (NY). 2016 Nov 7;6:422. doi: 10.7916/D8M32W3H.
Paton DM. Deutetrabenazine: treatment of hyperkinetic aspects of Huntington's disease, tardive dyskinesia and Tourette syndrome. Drugs Today (Barc). 2017 Feb;53(2):89-102. doi: 10.1358/dot.2017.53.2.2589164.
Gilbert DL, Dubow JS, Cunniff TM, Wanaski SP, Atkinson SD, Mahableshwarkar AR. Ecopipam for Tourette syndrome: a randomized trial. Pediatrics. 2023 Feb 1;151(2):e2022059574. doi: 10.1542/peds.2022-059574.
Gilbert DL, Murphy TK, Jankovic J, Budman CL, Black KJ, Kurlan RM, Coffman KA, McCracken JT, Juncos J, Grant JE, Chipkin RE. Ecopipam, a D1 receptor antagonist, for treatment of tourette syndrome in children: a randomized, placebo-controlled crossover study. Mov Disord. 2018 Aug;33(8):1272-1280. doi: 10.1002/mds.27457.
Abi-Jaoude E, Bhikram T, Parveen F, Levenbach J, Lafreniere-Roula M, Sandor P. A double-blind, randomized, controlled crossover trial of cannabis in adults with Tourette syndrome. Cannabis Cannabinoid Res. 2023 Oct;8(5):835-845. doi: 10.1089/can.2022.0091.
Pandey S, Srivanitchapoom P, Kirubakaran R, Berman BD. Botulinum toxin for motor and phonic tics in Tourette's syndrome. Cochrane Database Syst Rev. 2018 Jan 5;1(1):CD012285. doi: 10.1002/14651858.CD012285.pub2.
Moretti A. Is botulinum toxin effective and safe for motor and phonic tics in patients affected by Tourette syndrome? A Cochrane Review summary with commentary. Dev Med Child Neurol. 2020 Mar;62(3):274-276. doi: 10.1111/dmcn.14472.
Aguirregomozcorta M, Pagonabarraga J, Diaz-Manera J, Pascual-Sedano B, Gironell A, Kulisevsky J. Efficacy of botulinum toxin in severe Tourette syndrome with dystonic tics involving the neck. Parkinsonism Relat Disord. 2008;14(5):443-5. doi: 10.1016/j.parkreldis.2007.10.007.
Tao D, Zhong T, Ma S, Li J, Li X. Randomized controlled clinical trial comparing the efficacy and tolerability of aripiprazole and sodium valproate in the treatment of Tourette syndrome. Ann Gen Psychiatry. 2019 Oct 10;18:24. doi: 10.1186/s12991-019-0245-3.
Yang CS, Zhang LL, Lin YZ, Guo Q. Sodium valproate for the treatment of Tourette׳s syndrome in children: a systematic review and meta-analysis. Psychiatry Res. 2015 Apr 30;226(2-3):411-7. doi: 10.1016/j.psychres.2014.08.058.
Rizzo R, Gulisano M, Calì PV, Curatolo P. Tourette syndrome and comorbid ADHD: current pharmacological treatment options. Eur J Paediatr Neurol. 2013 Sep;17(5):421-8. doi: 10.1016/j.ejpn.2013.01.005.
Jaffe RJ, Coffey BJ. Pharmacologic treatment of Comorbid attention-deficit/hyperactivity disorder and Tourette and tic disorders. Child Adolesc Psychiatr Clin N Am. 2022 Jul;31(3):469-477. doi: 10.1016/j.chc.2022.03.004.
Neri V, Cardona F. Clinical pharmacology of comorbid obsessive-compulsive disorder in Tourette syndrome. Int Rev Neurobiol. 2013;112:391-414. doi: 10.1016/B978-0-12-411546-0.00013-5.
Rothenberger A, Roessner V. Psychopharmacotherapy of obsessive-compulsive symptoms within the framework of Tourette syndrome. Curr Neuropharmacol. 2019;17(8):703-709. doi: 10.2174/1570159X16666180828095131.
Porta M, Sassi M, Menghetti C, Servello D. The need for a proper definition of a "treatment refractoriness" in tourette syndrome. Front Integr Neurosci. 2011 Jun 6;5:22. doi: 10.3389/fnint.2011.00022.
Porta M, Cavanna AE, Zekaj E, D'Adda F, Servello D. Selection of patients with Tourette syndrome for deep brain stimulation surgery. Behav Neurol. 2013;27(1):125-31. doi: 10.3233/BEN-120288.
Schrock LE, Mink JW, Woods DW, Porta M, Servello D, Visser-Vandewalle V, et al.; Tourette Syndrome Association International Deep Brain Stimulation (DBS) Database and Registry Study Group. Tourette syndrome deep brain stimulation: a review and updated recommendations. Mov Disord. 2015 Apr;30(4):448-71. doi: 10.1002/mds.26094.
Dehning S, Leitner B, Schennach R, Müller N, Bötzel K, Obermeier M, Mehrkens JH. Functional outcome and quality of life in Tourette's syndrome after deep brain stimulation of the posteroventrolateral globus pallidus internus: long-term follow-up. World J Biol Psychiatry. 2014 Jan;15(1):66-75. doi: 10.3109/15622975.2013.849004.
Zhang JG, Ge Y, Stead M, Zhang K, Yan SS, Hu W, Meng FG. Long-term outcome of globus pallidus internus deep brain stimulation in patients with Tourette syndrome. Mayo Clin Proc. 2014 Nov;89(11):1506-14. doi: 10.1016/j.mayocp.2014.05.019.
Müller-Vahl KR. Surgical treatment of Tourette syndrome. Neurosci Biobehav Rev. 2013 Jul;37(6):1178-85. doi: 10.1016/j.neubiorev.2012.09.012.
Piedad JC, Rickards HE, Cavanna AE. What patients with gilles de la tourette syndrome should be treated with deep brain stimulation and what is the best target? Neurosurgery. 2012 Jul;71(1):173-92. doi: 10.1227/NEU.0b013e3182535a00.
Andrade P, Visser-Vandewalle V. DBS in Tourette syndrome: where are we standing now? J Neural Transm (Vienna). 2016 Jul;123(7):791-796. doi: 10.1007/s00702-016-1569-7.
Baldermann JC, Schüller T, Huys D, Becker I, Timmermann L, Jessen F, Visser-Vandewalle V, Kuhn J. Deep brain stimulation for Tourette-syndrome: a systematic review and meta-analysis. Brain Stimul. 2016 Mar-Apr;9(2):296-304. doi: 10.1016/j.brs.2015.11.005.
Xu W, Zhang C, Deeb W, Patel B, Wu Y, Voon V, Okun MS, Sun B. Deep brain stimulation for Tourette's syndrome. Transl Neurodegener. 2020 Jan 13;9:4. doi: 10.1186/s40035-020-0183-7.
Rusheen AE, Rojas-Cabrera J, Goyal A, Shin H, Yuen J, Jang DP, Bennet KE, Blaha CD, Lee KH, Oh Y. Deep brain stimulation alleviates tics in Tourette syndrome via striatal dopamine transmission. Brain. 2023 Oct 3;146(10):4174-4190. doi: 10.1093/brain/awad142.
Deeb W, Malaty I. Deep brain stimulation for Tourette syndrome: potential role in the pediatric population. J Child Neurol. 2020 Feb;35(2):155-165. doi: 10.1177/0883073819872620.
Bodeck S, Lappe C, Evers S. Tic-reducing effects of music in patients with Tourette's syndrome: self-reported and objective analysis. J Neurol Sci. 2015 May 15;352(1-2):41-7. doi: 10.1016/j.jns.2015.03.016.
Reilly C, Grant M, Bennett S, Murphy T, Heyman I. Review: Physical exercise in Tourette syndrome - a systematic review. Child Adolesc Ment Health. 2019 Feb;24(1):3-11. doi: 10.1111/camh.12263.
Pringsheim T, Nosratmirshekarlou E, Doja A, Martino D. Physical activity, sleep and neuropsychiatric symptom severity in children with tourette syndrome. Eur Child Adolesc Psychiatry. 2021 May;30(5):711-719. doi: 10.1007/s00787-020-01552-1.
Ricketts EJ, Burgess HJ, Montalbano GE, Coles ME, McGuire JF, Thamrin H, McMakin DL, McCracken JT, Carskadon MA, Piacentini J, Colwell CS. Morning light therapy in adults with Tourette's disorder. J Neurol. 2022 Jan;269(1):399-410. doi: 10.1007/s00415-021-10645-z.
Maiquez BM, Smith C, Dyke K, Chou CP, Kasbia B, McCready C, Wright H, Jackson JK, Farr I, Badinger E, Jackson GM, Jackson SR. A double-blind, sham-controlled, trial of home-administered rhythmic 10-Hz median nerve stimulation for the reduction of tics, and suppression of the urge-to-tic, in individuals with Tourette syndrome and chronic tic disorder. J Neuropsychol. 2023 Sep;17(3):540-563. doi: 10.1111/jnp.12313.
Dyke K, Jackson G, Jackson S. Non-invasive brain stimulation as therapy: systematic review and recommendations with a focus on the treatment of Tourette syndrome. Exp Brain Res. 2022 Feb;240(2):341-363. doi: 10.1007/s00221-021-06229-y.
Grados M, Huselid R, Duque-Serrano L. Transcranial magnetic stimulation in Tourette syndrome: a historical perspective, its current use and the influence of comorbidities in treatment response. Brain Sci. 2018 Jul 6;8(7):129. doi: 10.3390/brainsci8070129.
Bloch Y, Arad S, Levkovitz Y. Deep TMS add-on treatment for intractable Tourette syndrome: a feasibility study. World J Biol Psychiatry. 2016 Oct;17(7):557-61. doi: 10.3109/15622975.2014.964767.
Kahl CK, Kirton A, Pringsheim T, Croarkin PE, Zewdie E, Swansburg R, Wrightson J, Langevin LM, Macmaster FP. Bilateral transcranial magnetic stimulation of the supplementary motor area in children with Tourette syndrome. Dev Med Child Neurol. 2021 Jul;63(7):808-815. doi: 10.1111/dmcn.14828.