Introduction
Hypertrophic cardiomyopathy (HCM) is a genetic disease (sarcomeric protein mutation) with autosomal dominant transmission [1]. The disease was described 55 years ago by Braunwald E. and colleagues [2] as idiopathic hypertrophic subaortic stenosis. The pathology can present diverse in terms of medical history and clinical course, ranging from the development of acute heart failure or even sudden death, while other patients may remain asymptomatic throughout life. At the same time, there is a lack of correlation between the genotype and the phenotype of the disease. Thus, in a family, in two members with the same genetic abnormality, one may present a clinical manifestation of severe heart failure, the other being asymptomatic.
There are two main imaging morphological features of HCM [1]:
Left ventricular (LV) hypertrophy (>15 mm), with risk of sudden death (as ventricular thickening progresses) and heart failure (predominantly diastolic dysfunction) [1, 7].
LV outflow tract (LVOT) obstruction, usually affecting 70% of patients (gradient >30 mm Hg), present at rest or not being silent at physical exertion, medication administration or change of body position [1, 8].
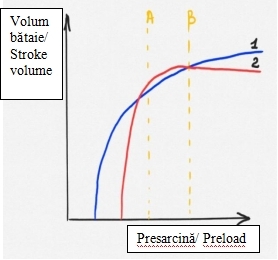
For clarity, the schematic imaging visualization of the LVOT on transesophageal echocardiography (130°) is depicted in Figure 1: for a healthy heart (Fig. 1A) and for HCM (Fig. 1B). The obvious abnormality observed in Fig. 1B is the thickened septum, which is responsible for narrowing the LVOT and which, as a result, produces an increased pressure gradient between the LV and the aorta. As a result of the acceleration of the blood flow through the narrowed area, the anterior mitral cusp retracts towards the septum, by the Venturi effect. The phenomenon is also facilitated by the fact that patients with HCM also show narrowing of the aorto-mitral angle compared to healthy people.
In descending order of importance, the risk factors for sudden death of the patient with HCM are [1]:
Major factors: anamnesis of hereditary-collateral sudden death, unexplained syncope, repetitive unsustainable ventricular tachycardia (multiple episodes), massive LV hypertrophy (>30 mmHg), LV apical aneurysm, extensive nuclear magnetic resonance fibrosis (MRI) (late gadolinium enhancement), end-stage heart failure (ejection fraction (FE) <50%);
Potential mediators of risk: hypotensive response to physical exertion, obstruction of the LVOT at rest.
The stratification of patients with HCM according to the degree of risk, with the early initiation of HCM management protocols, has contributed to the improvement of morbidity and mortality. Thus, HCM-associated mortality was reduced by 90% compared to data reported 35 years ago [1]. The management of patients with HCM has clearly evolved in the last decade, offering:
Implantable cardioverter defibrillator in order to prevent sudden death caused by ventricular tachycardia;
Drug treatment (beta-blockers or calcium channel blockers) for patients who develop progressive heart failure;
For obstructive HCM (90%) septal myomectomy: surgical or alcohol ablation;
For non-obstructive HCM (10%): heart transplant;
For atrial fibrillation: anticoagulants and antiarrhythmics (or catheter ablation);
A special challenge is the patient with HCM (both diagnosed and known, but especially asymptomatic), who is admitted to the anesthesiology service for the non-cardiac surgery.
Clinical case
Patient description. We report a case of a 34-year-old male (64 kg, 173 cm, 1,76 m2) who comes to see a cardiologist with complaints of moderate-intensity dyspnea, general weakness, and fatigue.
History of the disease. The patient has been ill for about a week having pronounced dyspnea at rest, orthopnea, and general weakness – the complaints with which he went to see the family doctor. Pulmonary tomography, realized 24 hours after the consultation, revealed suspicious signs for SARS-CoV-2 infection, but the real-time reverse-transcriptase polymerase chain reaction (rRT-PCR) test for COVID-19 infection was negative (27.11.2020). Subsequently, the patient is admitted to one of the municipal hospitals, where repeated testing for COVID-19 infection shows a positive result (28.11.2020) and the patient-relevant treatment is initiated. The COVID-19 infection test is repeated in a few days and a negative result is obtained (30.11.2020). Ultrasound examination of the internal organs found fluid collections in the projections of the right (290 ml) and left (300 ml) costodiaphragmatic sinuses, also signs of chronic cholecystopancreatitis. Also, the echocardiographic examination (ECOCG) of the heart realized at the same public hospital, revealed dilation of all heart cavities, moderate to severe (grade III) mitral valve insufficiency (MtV), moderate to severe (grade II-III) tricuspid valve (TrV) insufficiency, severe pulmonary hypertension (PH), with an ejection fraction (EF) of about 27%.
According to the patient, he has not been in contact with people suffering from infectious diseases and has not travelled abroad in the last 2 weeks.
Physical examination. The patient's general condition is stable. Pale, clean skin, no peripheral oedema on the legs. Auscultation reveals enhanced breathing sounds, rhythmic heart sounds, tachycardia 100 bpm. Blood pressure (BP) 119/80 mmHg. Soft, symmetric, and non-tender abdomen. Bowel sounds are present and normoactive in all four quadrants. Preserved diuresis and painless urination.
Paraclinic investigations results. Laboratory tests: only aspartate aminotransferase (TGO/AST) elevation (38,5 U/L). On the electrocardiogram (ECG): regular sinus rhythm, right axis deviation, posterior hemiblock of the left bundle branch, complete block of the right bundle branch. The ECOCG Doppler realized in our clinic finds considerable dilation of the left side of the heart, moderate dilation of the right side, also dilation of the trunk of the pulmonary artery and its branches (left branch 17 mm, right branch 17 mm), severe LV myocardial hypertrophy. Severe diffuse LV myocardial hypokinesia with 20% EF, severely reduced LV longitudinal systolic contraction function (Table 1).
Presumptive diagnosis: Congestive heart failure. Post-inflammatory cardiomyopathy. Myocarditis. Cardiac MRI was recommended to confirm or rule out a diagnosis of hypertrophic cardiomyopathy or a storage disorder that mimic de phenotype of the HCM (amyloidosis, Fabry disease etc.).
Indicated treatment: limited fluid intake only up to 1.5 L in 24 hours (summing all fluids administered), diuretics (spironolactone 50 mg, 1 tablet and torasemide 5 mg, 1 tablet both in the morning before breakfast), beta-blockers (carvedilol 6,25 mg, 1 tablet twice in 24 hours), medication with a positive impact on the body's energy metabolism (meldonium 500 mg, 1 tablet twice in 24 hours, during 3 months), anticoagulants (rivaroxaban 20 mg, 1 tablet every evening during 1 month). Blood biochemical tests (urea, creatinine) were taken. Cardiac MRI and repeated consultation after 2 weeks were indicated.
After one month, at the repeated consultation of the cardiologist, the patient does not present any complaints, except for a general weakness. Objective clinical examination shows a stable general condition, pale, clean skin, and no peripheral oedema on the legs. Enhanced bilateral pulmonary sounds at lung auscultation, rhythmic heart sounds with heart rate 80 bpm, BP 100/70 mmHg. Soft, symmetric, and non-tender abdomen. Present bowel sounds, normoactive in all four quadrants. Preserved diuresis, painless urination.
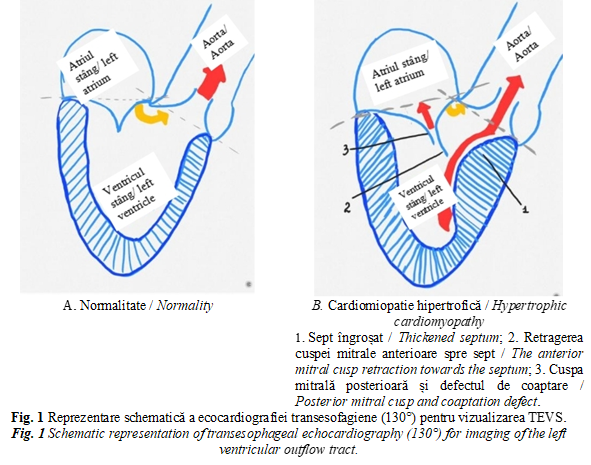
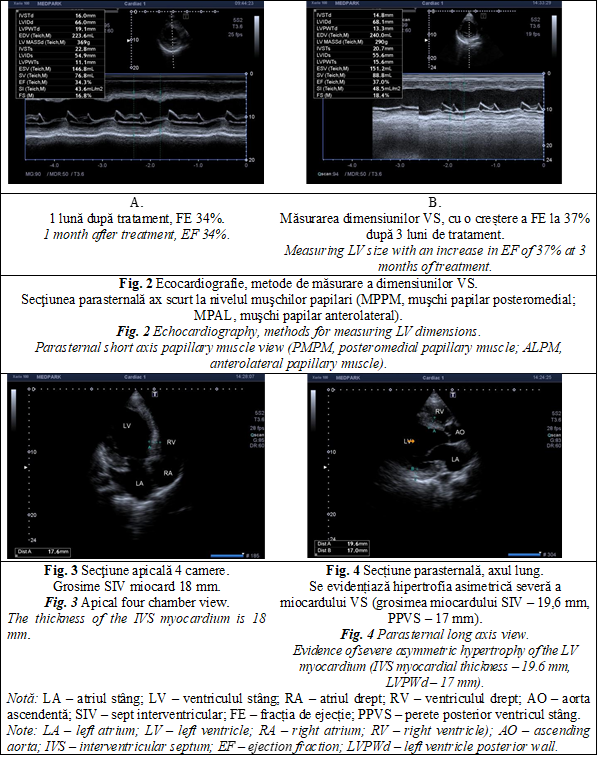
On echocardiographic examination, in comparison with the imaging picture 5 weeks ago, there was a slight positive dynamic. Thus, ECOCG Doppler finds a reduction in the size (from 51 to 49 mm) and the volume of the left atrium (from 97 to 87 ml, volume index from 56 to 47 ml/ m2), a reduction in the size of the LV (DTD from 70 to 67 mm, VTD from 253 to 234 ml), increased EF (from 20 to 34%), decrease in right ventricle size (from 30 to 25 mm) and right atrium (from 54 to 50 mm), decrease in PH from severe (78-80 mm Hg) to moderate (35 mm Hg) (Figure 2). Also, the pulsatile and continuous Doppler examination detects the reduction of the velocities in the projection of the valves with amelioration of regurgitation at MtV (from III to II), aortic valve (from I to zero), TrV (from III to II), pulmonary valve (from to II to I) (Table 1). ECOCG attests a type 3 (restrictive) diastolic dysfunction revealed by high LV filling pressures and assessed by estimating the transmittance flow with the determination of the E and A waves, E/ A ratio (Table 1). Furthermore, at 3 months from the first consultation in our clinic, there is observed a clear improvement in the patient's condition and cardiac function with a 37% increase in EF (Table 1, Figure 2B). At the same time, severe asymmetric LV myocardial hypertrophy is evident in both the 4-chamber apical section and the long-axis parasternal section (Figures 3 and 4).
Cardiac MRI-3T (18.12.2020) reveals severe diffuse non-ischemic fibrosis lesions of the LV myocardium, severe LV myocardial hypertrophy, predominantly IVS (18 mm), severely dilated LV, with severe global systolic dysfunction (estimated LV volume: systole 315 ml, diastole 375 ml, LV stroke volume 76 ml, LV EF 17%, cardiac output 6.3 L/min, lateral wall thickness 9 mm), slightly dilated RV with moderate global systolic dysfunction (estimated RV volume: systole 115 ml, diastole 169 ml, RV stroke volume 54 ml, RV EF 32%, lateral wall 7-8 mm), LA dilated moderately (MtV regurgitation fraction 29%, moderate degree), RA normal size. Aortic, TrV and pulmonary valve without abnormalities.
Thus, the established diagnosis: post-inflammatory cardiomyopathy. Myocarditis. Severe MtV insufficiency (grade III). Moderate to severe TrV insufficiency (degree II-III). Severe PH. Congestive heart failure NYHA class III, stage C (ACC/ AHA, American College of Cardiology and the American Heart Association).
To the prescribed earlier treatment was added the combined angiotensin receptor antagonist / neprilysin inhibitor (uperio 50 mg twice in 24 hours).
Clinical and paraclinical examination at more than 3 months distance from the first request for a cardiologist's consultation, there is concluded a clearly positive dynamic, with the LV geometry and performance remodeling (Table 1).
Tabelul 1. Rezultatele examenului ecocardiografic color Doppler în dinamică. Table 1. Dynamics of the Doppler echocardiographic examination results. | ||||
Parametri Parameters | 14.12.2020 | 28.01.2021 | 02.04.2021 | Valori de referință Reference values |
DTDVS / LVEDD | 70 mm | 67 mm | 60 mm | 35-56 mm |
DTSVS / LVESD | 62 mm | 57 mm | 49 mm | 25-41 mm |
SIV / IVS | 21 mm | 20 mm | 20 mm | 6-11 mm |
VTDVS / LVEDV | 253 ml | 234 ml | 180 ml | 53-156 ml |
VTSVS / LVESV | 196 ml | 159 ml | 112 ml | 23-76 ml |
FE / EF | 20 % | 34 % | 37 % | >50% |
PPVS / LVPWd | 17 mm | 17 mm | 17 mm | 6-11 mm |
E | - | 112 cm/ sec | 119 cm/ sec | – |
A | - | 84 cm/ sec | 94 cm/sec | – |
E/A | - | 1,3 | 1,3 | – |
Vmax VT / Vmax. TrV | 4,3 m/sec | 2,6 m/ sec | 2,3 m/ sec | – |
Funcție diastolică Diastolic function | 3 (restrictiv / restrictive) | 3 (restrictiv / restrictive) | 2 (afectată / affected) | – |
Gradul de insuficiență a valvelor cardiace / Degree of heart valve insufficiency | ||||
VM / MtV | III | II | II | – |
VAo / AoV | 0-I | - | - | – |
VT / TrV | II-III | I-II | II | – |
VP / PV | II | I | I | – |
PSAP / PASP | 70-80 mm Hg | 35 mm Hg | 30 mm Hg | – |
Notă: DTDVS – diametrul telediastolic al VS; DTSVS – diametrul telesistolic al VS; SIV – sept interventricular; VTDVS – volumul telediastolic al VS; VTSVS – volumul telesistolic al VS; FE – fracția de ejecție; PPVS – perete posterior ventricul stâng; undele E și A; raportul E/A – aprecierea debitului transmitral; VM – valva mitrală; VAo – valva aortică; VT – valva tricuspidă; VP – valva pulmonară; Vmax VT – viteza maximă valva tricuspidă; PSAP – presiunea sistolică în artera pulmonară. Note: LVEDD – LV end-diastolic diameter; LVESD – LV end-systolic diameter; IVS – interventricular septum; LVEDV LV – LV end-diastolic volume; LVESV – LV end-systolic volume; EF – ejection fraction; LVPWd – left ventricle posterior wall; E and A waves; E/A ratio – mitral flow velocity; MtV – mitral valve; AoV – aortic valve; TrV – tricuspid valve; PV – pulmonary valve; Vmax TrV – maximum velocity tricuspid valve; PASP – pulmonary artery systolic pressure. | ||||
Discussion
Only 10% of patients with HCM are clinically identifiable (60% of which are symptomatic and 40% asymptomatic). Most (90%) remain unidentified, carrying a disease without obvious symptoms that would lead them to the consultation of a medical specialist [1].
Even with a low prevalence in general population, patient with HCM also may present COVID-19 infection. At the same time, young otherwise healthy adult may develop COVID-19 associated myocarditis. What is interesting in the reported case is the fact that even silent during the acute phase of COVID-19, the acute cardiac failure symptoms precipitated far in the post-acute period. The presented case of acute cardiac failure in young male with no previous cardiac signs and symptoms is of interest because it debates clinical combinations that were not reported in literature previously: primary diagnosed HCM overlapped on the ongoing COVID-19 infection, isolated post-COVID-19 myocarditis, HCM combined with myocarditis and COVID-19 infection. Clinical importance of the case consists of sudden death risk and an eventual anaesthetic management in case of urgent or elective surgery.
Viewed through the perspective of an eventual perianesthetic management, the main problem related to the patient with asymptomatic HCM represents the identification of this ”silent clinical condition” during the preanesthetic visit. Therefore, in case of any symptoms of exercise intolerance in a young person, especially associated with systolic murmur, specialist should be concerned about these clinical signs and try to rule out a possible HCM by recommending an echocardiographic examination.
Given that it is a genetically determined pathology, HCM means LV hypertrophy under normal conditions, without pre-existing functional overload. Therefore, HCM should not be confused with hypertrophy caused by functional ventricular overload found in systemic hypertension or the aortic valve stenosis (AoV). Thus, in the case of hypertension also known as high blood pressure, increased systemic vascular resistance imposes difficulties for LV blood ejection during systole. In case of AoV stenosis, there is a AoV resistance per se, due to the reduction of the surface of the AoV orifice. Both high blood pressure and AoV stenosis result from overload of the LV and, as a result, it compensates by hypertrophy [9, 10].
For the diagnosis of HCM is necessary to measure the thickness of the IVS and the inferolateral wall in parasternal long-axis and short axis views. If one of the measurements exceeds 15 mm, the problem of hypertrophy is raised and if this cannot be explained by hypertension or AoV stenosis, then the clinical condition will be investigated for HCM. At the same time, the athletic heart often has imaging manifestations of physiological hypertrophy that must be differentiated from HCM. Similarly, accumulation metabolic disorders and mitochondrial defects can lead to thickening of the ventricular wall, requiring them to be differentiated from HCM. Clinical judgment and differentiation analysis will be done in the light of the following suggestive for cardiomyopathy criteria: (1) Hyperdynamic LV; (2) Severe septal hypertrophy; (3) LVOT obstruction; (4) Lowered LV [9, 10].
Tabelul 2. Maladii ce decurg clinic cu fenotip de CH [9]. Table 2.Diseases that mimic de phenotype of the HCM [9]. | ||
Fenotip / Phenotype | Indiciu fenotipic / Phenotypic clue | |
1. | Glicogenoza AMPK-mediată AMPK-mediated glycogen storage | Funcționalitate sistolică normală sau redusă a VS, tipar de pre-excitare. Normal or reduced LV systolic function, pre-excitation pattern. |
2. | Boala Pompe (glicogenoza tip II) Pompe disease (Glycogen storage disease type II) | Afecțiune neuromusculară rară, cu transmitere autosomal recesivă, maladii poliorganice, patern de preexcitare. Rare autosomal recessive skeletal muscle weakness, multiorgan disease, pre-excitation pattern. |
3. | Boala Anderson-Fabry (angiokeratoza difuza) Anderson-Fabry disease (diffuse angiokeratosis) | X-linkată, multisistemica implicând inclusiv pielea, rinichii și nervii periferici. X-linked, multisystemic disease involving skin, kidney and peripheral nerves. |
4. | Boala Danon Danon disease | X-linkată dominant, miopatie a mușchilor scheletici, afectarea intelectului, scurtarea PR la traseul ECG, valori CK elevate. X-linked dominant skeletal disorder, with impaired intellect, shortened PR on ECG, high CK values. |
5. | Amiloidoza Amyloidosis | Voltaj QRS redus, implicarea mai multor organe, LGE subendotelial. Low QRS voltage, involvement of several organs, subendothelial LGE. |
6. | Sindromul Kearns-Sayre Kearns-Sayre syndrome | Maladie multisistemică. Multisystemic disease. |
7. | Ataxia Friedreich Friedreich ataxia | Autosomal recesiv, neurodegenerare. Autosomal recessive, neurodegeneration. |
8. | Distrofie miotonică Myotonic dystrophy | Miotonie, distrofie musculară, cataractă, chelie frontală. Myotonia, myotonic dystrophy, cataract, frontal baldness. |
9. | Sindroamele Noonan / LEOPARD (RASopatii) Noonan / LEOPARD syndromes (RASopathies) | Malformații cardiace congenitale, anomalii dismorfogenetice, efelide, maculele "cafe-au-lait" pe față, gât și pe jumătatea superioară a trunchiului. Cardiac congenital malformations, lentigines, "cafe-au-lait" spots mostly on the face, neck, and upper body. |
10. | Boala Neimann-Pick Neimann-Pick disease | Maladie neurodegenerativă autosomal recesivă. Autosomal recessive neurodegenerative disease. |
11. | Boala Refsum Refsum disease | Retinita pigmentoasă, ataxie și neuropatie periferică. Retinitis pigmentosa, ataxia and peripheral neuropathy. |
12. | Surditate Deafness | Surditate autosomal dominantă. Autosomal dominant deafness. |
Notă: AMPK – protein kinaza AMP-activată; ECG – electrocardiogramă; CK – creatin kinaza; LGE – engl. late gadolinium enhancement imaging, LEOPARD (acronim introdus în 1968): „Lentigines“ (efelide), anomalii de conducere pe ECG, hipertelorism Ocular, stenoză Pulmonară, Anomalii genitale, Retard psihic și Dificultăți ale auzului). Note: AMPK – AMP-activated protein kinase; ECG – electrocardiogram, CK – creatin kinase; LGE – late gadolinium enhancement imaging, LEOPARD (acronym introduced in 1968: Lentigines, ECG abnormalities, Ocular hypertelorism, Pulmonary stenosis, Abnormal Genitalia, Retarded growth and Deafness. | ||
The clinical diagnosis of HCM is established based on a hypertrophied and undilated LV attested by echocardiographic examination or MRI, in the absence of other metabolic, cardiac, systemic diseases or syndromes [3, 10]. Also, a differentiated diagnosis will be made with a series of diseases that mimic de phenotype of the HCM (Table 2) [9].
The epidemiological study of Maron B. J. and colleagues [4] based on echocardiographic screening of the population reported prevalences of 1 case of HCM in 500 people in the general population. Semsarian C.'s group [5] detects higher prevalences (echocardiography in combination with genetic tests): 1 case of HCM in 200 people between the family members of a patient with HCM. In most clinically diagnosed cases, the thickness of the LV wall was 15 mm or more (average 21 mm), in some cases there was a massive thickening (30-50 mm) [1, 4, 10]. LV thickness wall at the limit of 13-14 mm requires a differential diagnosis with systemic hypertension or athletic physiological cord. Therefore, it is necessary to monitor in the HCM spectrum both the patient with thickening of the LV wall and in case of a heart within normal limits found in a carrier of the HCM genetic mutation [1, 3].What is certain is that in HCM, the degree of LV wall hypertrophy is directly proportional to the risk of sudden death [6] but does not correlate with the rapid progression of heart failure [1, 4]. For the patient with positive collateral-hereditary history of HCM, echocardiographic screening is indicated every 12-18 months between 18-21 years old, because HCM develops rapidly during the period of intensive growth and maturation, then the patients come to be reassessed every 5 years [7].
HCM can be clinically manifested by obstruction, 70% of patients showing mechanical impedance at LVOT (gradient ≥30 mm Hg), estimated at rest or physical effort [8]. The subaortic gradient is dynamic and vary with change in physiological stress conditions (increased and associated with reduced ventricular volume due to dehydration, alcohol, or food consumption, or changing posture from sitting to standing), these changes are often responsible of fluctuations in symptoms during the day [1].
LVOT obstruction is caused by systolic movement of the anterior mitral valve and contact with the septum due to stream flow, also resulting in mitral regurgitation [1,4,8]. Abnormal congenital insertion of papillary muscle directly into the MtV (without interposition of the cords) is occasionally responsible for medioventricular muscle obstruction and is an indication for planning invasive treatment strategies [3].
Prophylactic installation of implantable cardioverter defibrillator to prevent sudden death from ventricular tachycardia is indicated for young and middle-aged people who have at least one major risk factor or several minor risk factors for sudden death.
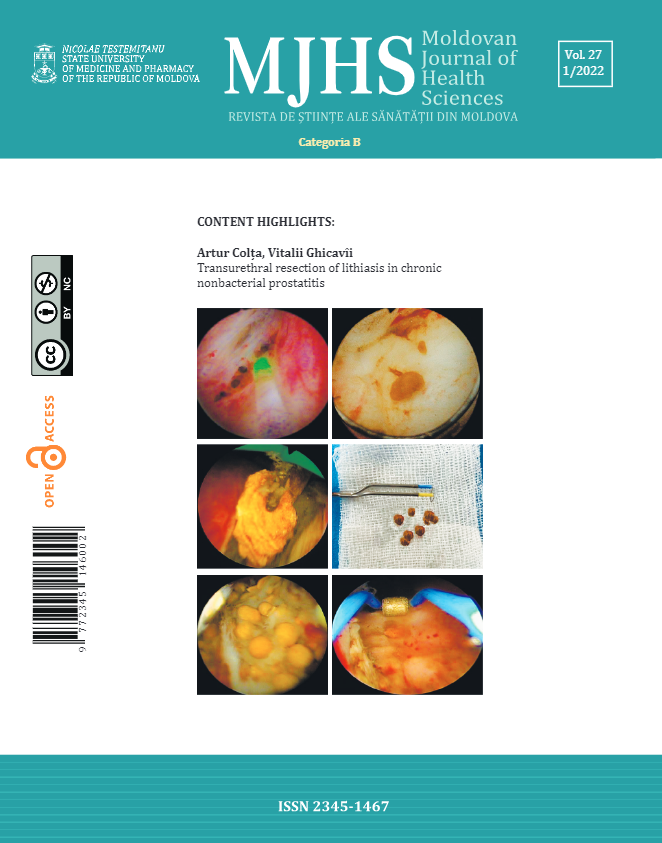
If the suspicion of HCM is confirmed, the patient's perianesthetic management will radically change. First of all, it will be avoided all of the dangerous situations that could facilitate (hypovolemia, acute vasodilation) or directly aggravate (inotropic medication) the obstruction of LVOT. For example, in case of a known with HCM patient, in order to avoid hypovolemia due to acute vasoplegia spinal anesthesia is not indicated, general anesthesia with sequential induction being the best choice. Secondly, volume therapy should be judiciously titrated, given that these patients have a diastolic dysfunction. Thus, it is indicated the invasive monitoring of blood pressure, with a slower induction, but with a sufficient anesthetic depth for the airway instrumentation and, as a result, a mild hemodynamic response. Due to myocardial hypertrophy, patients with HCM have an abnormal curve of cardiovascular function (Figure 5, curve 2), with both a narrowed corridor of values in which the heart works within acceptable limits (Figure 5, corridor AB) and a narrowed edge of fluid therapy.
|
Fig. 5 Curba funcției cardiovasculare. Fig. 5 Curve of cardiovascular function. |
In other words, if the preload decreases (bleeding, acute installed vasodilation, etc.), the stroke volume will decrease rapidly.On the other hand, in case of volume overload, the plateau of the cardiovascular function curve of these patients can be reached quickly, with the risk of congestion and pulmonary oedema (frequently observed postoperatively in this group of patients).Therefore, intra-anesthetic fluid titration will be performed based on stroke volume, avoiding both hypovolemia and volume overload. Thus, after each fluid administration, by the help of transesophageal echocardiography, it should be analyzed the improvement of the stroke volume (responsive to fluids) or the ceiling effect of the improvement (restriction of administration).
For such a case management it is imperative to understand the pathophysiology of HCM and to maintain the hemodynamic objectives. Key points of the anesthetic management for the patients with HCM are: a) maintenance of sinus rhythm and immediate management of arrhythmias b) adequate maintenance of preload; c) avoidance of acute vasodilation; d) management of hypotension with vasopressors; e) improvement of cardiac contractility; f) management of congestive heart failure.
Conclusions
HCM is a common congenital heart disease. HCM treatment has evolved a lot in the last decade and offers the opportunity to reduce symptoms by extending life expectancy for most patients. However, a large proportion of patients remain undiagnosed. Managing anesthesia in a patient with HCM is a challenge for the anesthesiologist and requires an advanced cardiac monitoring system, involving special warnings: avoidance of pulmonary oedema (due to severe diastolic dysfunction) and hemodynamic instability (due to obstruction of the LVOT).
Note on patient informed consent
After detailed explanations and assurance that our attitude towards him will not change regardless of his decision, the patient signed the informed consent for the publication of the clinical case presentation based on his medical record, respecting the right to confidentiality.
Competing interests
None declared
Authors' contribution
All authors contributed equally to the development and writing of the manuscript. The authors read and approved the final version of the manuscript.
Authors’ ORCID IDs
Natalia Belîi - https://orcid.org/0000-0002-2351-0279
References
- Maron B. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med, 2018; 379 (7): 655-668.
Braunwald E., Lambrew C., Rockoff S. et al. Idiopathic hypertrophic subaortic stenosis. I. A description of the disease based on an analysis of 64 patients. Circulation, 1964; 29 (4): 3-119.
Maron B., Maron M. The remarkable 50 years of imaging in HCM and how it has changed diagnosis and management: from M-mode echocardiography to CMR. JACC Cardiovasc Imaging, 2016; 9 (7): 858-872.
Maron B., Ommen S., Semsarian C. et al. Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol, 2014; 64 (11): 83-99.
Semsarian C., Ingles J., Maron M. et al. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol, 2015; 65 (12): 1249-1254.
Spirito P., Bellone P., Harris K. et al. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med, 2000; 86 (6): 1778-1785.
Maron M., Maron B. Clinical impact of contemporary cardiovascular magnetic resonance imaging in hypertrophic cardiomyopathy. Circulation, 2015; 132 (4): 292-298.
Maron M., Olivotto I., Zenovich A. et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation, 2006; 114 (21): 2232-2239.
Marian A., Braunwald E. Hypertrophic cardiomyopathy genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circulation Research, 2017; 121 (7): 749-770.
Protocol clinic național nr. 248: Cardiomiopatia hipertrofică. 2016. (http://89.32.227.76/_files/15214-PCN-248%2520Cardiomiopatia%2520hipertr…) (vizitat la 04.04.2021).