Introduction
Guided bone regeneration is a surgical method that allows obtaining a new bone in places of atrophy of the maxillary bone. The technique aims to restore sufficient bone volume to insert dental implants in an adequate way. The integration of the graft or the augmented bone under the mucosal flap and the primary healing of the soft tissues are essential conditions for preventing the exposure of the regeneration site and infectious complications that inevitably lead to the failure of the Guided Bone Regeneration (GBR) procedure. In the opinion of Thoma et al. the exposure of the membrane in the oral cavity has a significant impact on the overall result of the surgical procedure, leading to a reduction in the height of the augmentation between 40% and 60% [1, 2].
Wang and Boyapati introduced 4 minimal key principles that must be respected for GBR surgery to be successful: primary wound healing; angiogenesis; blood clot stability and maintaining augmentation space [3]. To ensure primary wound healing, flap shape and mobilization techniques play a crucial role in achieving complete closure of the augmented site. According to the authors Machtei et al. [4], these principles must be respected especially when using the GBR technique and performing vertical augmentations in the posterior area of the mandible, where the soft tissues are thin, and the edges of the flaps must be stretched. In these areas, the exposure of the membrane occurs most frequently [5, 6]. Therefore, optimal soft tissue management is required to achieve tension-free primary flap closure and to prevent infection of the membrane and augmentation material [7].
Burkhardt et al. reported a risk of dehiscence of 10% if the flap tension before suturing is less than 10 g and increases substantially to 40-100% if the flap tension exceeds 10 g [8]. All flaps sutured at more than 25.5 g show dehiscence, a low-tension flap suture can be achieved by combining an external-internal suspended suture, which greatly reduces tension at the flap edge, sutured with a second tension-free suture closer to the edge wounds [8]. Suture material and proper wound management with release incisions are also of considerable importance.
The shape of the flap is key to reducing post-bone augmentation complications. Zazou et al. recommended that for the coronal advance, it is necessary to perform a deperiosteal incision at the junction of the fixed and mobile mucosa in the case of the vestibular flap and to carry out tight muscle debridement in the case of the lingual flap [9]. The author argues this modification of the incision provides a larger keratinized gingival sleeve to the lingual flap. Another important factor highlighted by Kim et al. proposes to make the flap without discharge incisions, they explain this particular approach preserves vascularization and venous return supply of the flap [2].
Dehiscence is known to be a major problem in bone regeneration surgery, according to authors such as Canullo et al. the important factor is not the technique of performing bone regeneration, but the shape and type of mucogingival flap prepared and advanced coronally, which will cover the regeneration [10]. Other authors such as Gallo et al. in a study conducted in 2019 on 80 cases obtained about 68% complications with exposure; they believe that the separation device is responsible for the occurrence of dehiscence [11]. By simply using collagen membranes over the titan devices, Urban et al. significantly decreased the rate of dehiscence to 3% of 65 sites undergoing regeneration [12]. Lately there has been increased emphasis on research that includes new techniques for the preparation of mucogingival flaps [13, 14].
Material and methods
This study presents the results of a cohort study, that includes 70 patients who underwent guided bone regeneration operations. The study was carried out in accordance with the ethical principles of the Declaration of Helsinki, approved by the Research Ethics Committee of Nicolae Testemițanu USMF, minutes No. 43 of 13.02.2020. The research is a multicenter one, conducted during the period 2019-2024, enrolled patients who applied for surgical treatment of guided bone augmentation within the Arsenie Guțan Department of Oro-Maxillo-Facial Surgery and Oral Implantology at the clinical base in Chisinau Municipal Dental Center and at the "MASTERDENT" SRL university clinical base.
The research included the use of the techniques for forming muco-periosteal flaps: the Modified Periosteal Releasing Incision (MPRI) according to the principle of the double flap technique (DF) and the coronal advanced lingual flap (CALF).
Patients were divided into two groups of 35 subjects each, as calculated using EpiInfo 7.2.2.6 program, "StatCalc – Sample Size and Power" compartment for analytical study based on the following parameters: 95.0% confidence interval a statistical power of 80.0%.
Inclusion criteria:
70 patients, aged between 20 and 60, both female and male, were included in the study. The subjects included in the research were patients who presented atrophy of the alveolar process, diagnosed clinically and paraclinically, making it impossible to insert a dental implant.
Exclusion criteria:
• Patients with coagulation disorders
• Pregnant and breastfeeding women
• Smokers more than five cigarettes a day
• Patients with unsatisfactory hygiene
• Patients with chronic otorhinolaryngological pathologies
• General pathologies such as: diabetes, glycemic values >7-8 mmol/l, oncological pathologies, diseases of the hematopoietic system, autoimmune diseases, liver cirrhosis, cardiac and respiratory pathologies.
I group – included patients to whom it was performed GBR using perforated titanium membrane;
II group – GBR was performed by using bioresorbable poly-4 hydroxybutyrate (P4HB) synthetic mesh.
The patients were evaluated periodically to monitor postoperative progress. Cases of dehiscence of the area related to augmentation site were recorded, measured and classified according to Fontana et al. [14]. Statistical results were generated and processed by the R Studio program. The following descriptive statistics were estimated for the numerical variables: minimum value, maximum value, mean value with standard deviation, median value with interquartile deviation (AI). For all the statistical tests applied in the study, the threshold value (p) was considered to be 0.05, completed with 95% confidence intervals for the relative frequencies.
Results
In our research, 70 patients underwent guided bone regeneration using resorbable polymer meshes and titanium membranes. All patients were operated with the creation of the mucogingival flap according to modern methods, using the "MPRI" technique to create vestibular flaps and the "CALF" technique for lingual flaps. Of these, 25 patients were operated on the lower jaw- mandible using the "MPRI" vestibular flap method and the lingual "CALF" method, another 45 patients were operated on the upper jaw using the "MPRI" vestibular flap method (see table 1).
Table 1. Demographic and clinical data | |
Number | 70 |
Gender: Male Female |
24 (34.3%) 46 (65.7%) |
Age | 53.1 (range, 27 – 60) |
Lower jaw operated patients "MPRI" vestibular flap combined with "CALF" technique for lingual flaps | 25 |
Upper jaw operated patients "MPRI" vestibular flap | 45 |
When analyzing the data, a total of 8 cases of complications with gingival dehiscence were registered , of which 4 cases were grade I dehiscence and 4 cases were grade II dehiscence according to Fontana.
In the control group where the titanium membrane was used, three cases of dehiscence were recorded, accounting for 5.7% (95% CI 1.21). Of these, grade I dehiscence – one case, located in the upper jaw where it was performed the „MPRI” flap and constituting 2.9% (95% CI 0.15,17). Grade II dehiscence occurred in two other cases, constituting 5.7% (95% CI 1.21), located on the mandible where the „MPRI” vestibular flap and „CLAF” lingual flap were performed. The remaining 32 cases in this group did not show any dehiscence, the ratio being 91.4% (95% CI 76 .98).
In the study group – five cases of dehiscence were observed as follows: grade I dehiscence were recorded in three clinical cases, constituting 8.6% (95% CI 2.2, 24), among which two cases were located in the mandible where was performed „MPRI” vestibular flap combine with „CLAF” lingual flap and one case in the upper jaw where it was performed the „MPRI” flap. Grade II dehiscence were in two cases – 5.7% (95% CI 1.21), both located in the mandible where it was performed the „MPRI” vestibular flap and „CLAF” lingual. The remaining 30 cases did not show any form of dehiscence, the proportion being 85.7% (95%CI 69, 95).
The management of dehiscence were grouped according to the degree of complications, as follows:
I group (control group) – dehiscence grade II were treated by partial removal of the membrane; the titanium membrane was performed by milling it, as recommended by Al-Ardah et al. [15] figure 1 (A).
II group (resorbable mesh group) – dehiscence grade I (4 cases, 5.7% (95% 1.8,15)) of gingival dehiscence were remedied with rinses with „Loroben” oral antiseptic solution and scheduled visits for local processing with 10% Polyvidon Iodine solution and 0.05% Chlorhexidine solution until the appearance of granulations and epithelization, the grade II dehiscence was treated by partial removal of the mesh by surgical scissors, figure 1(B).
It should be specified that all cases of dehiscence were treated early.
The remaining 62 respondents did not develop dehiscence complications and constituted 88.6% (95% CI 78, 95).
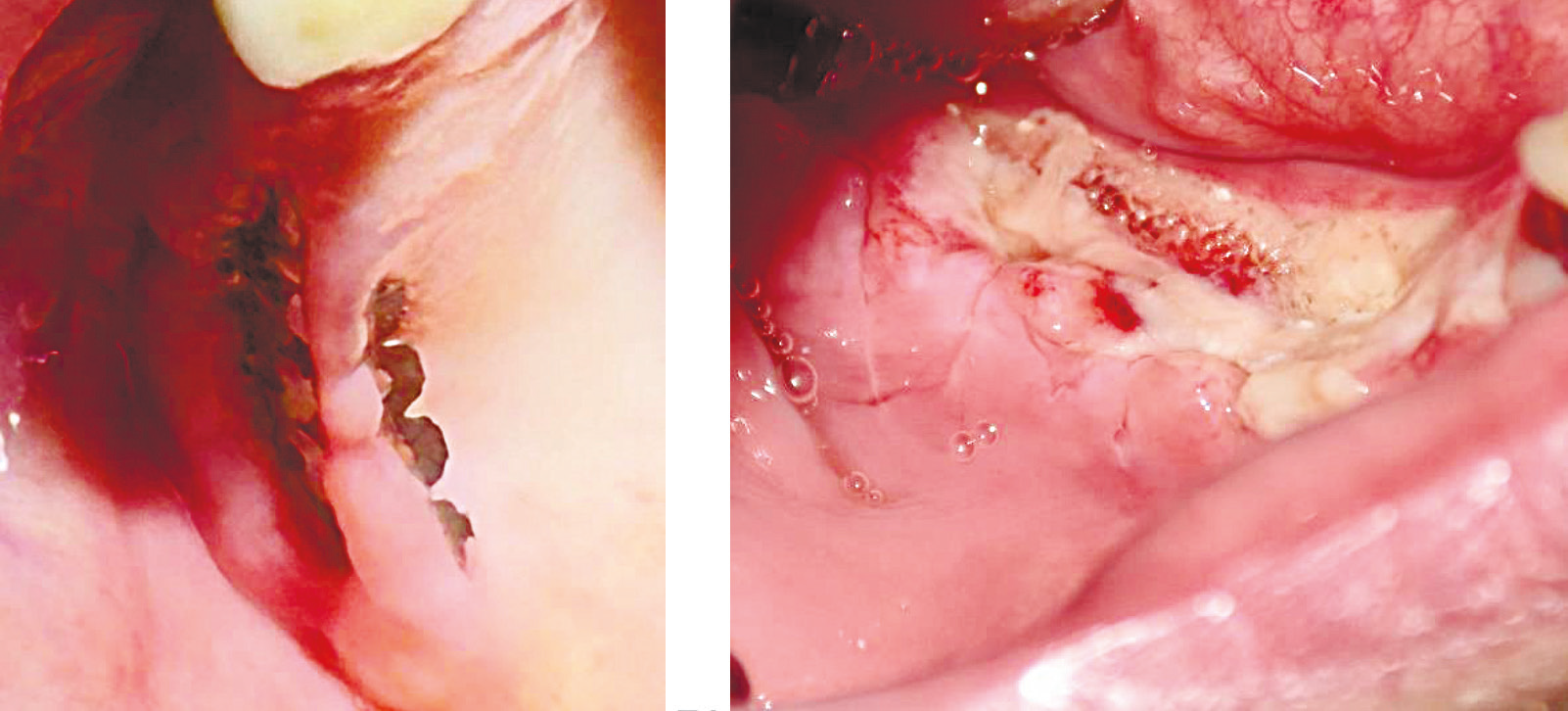 |
Fig. 1 Types of dehiscence, by following techniques: A. Dehiscence occurring after GBR treatment with titanium membrane in the upper right jaw. |
In table 2 we can see the general characteristics of the entire research group of dehiscence complications, and we can state that a total of 8 cases of dehiscence were recorded in the research.
Table 2. Representation of "Gingival dehiscence" values | ||
Variable | N = 70 | 95% CI |
Dehiscence grade |
| |
Grade 0 | 62 (88.6%) | 78%, 95% |
Grade I | 4 (5.7%) | 1.8%, 15% |
Grade II | 4 (5.7%) | 1.8%, 15% |
Note: N- number of patients, CI -confidence interval | ||
For categorical variables, absolute frequencies and relative frequencies were estimated, supplemented with 95% confidence intervals for relative frequencies. Visualization was performed using barplot graphics (bar diagram). Hypotheses testing was conducted using Pearson's Chi-square test (Monte Carlo variant with 10,000 repetitions). In the study, the dehiscence association between the performed bone regeneration techniques and the appearance of dehiscence was evaluated (see figure 2). The null hypothesis formulated was that there is no association between the applied technique and the appearance of gingival dehiscence, the alternative hypothesis being the presence of the association.
Applying the χ2 test (Monte Carlo variant) = 1.06, p = 0.84, which means that there are no significant statistical differences, the result being unchanged even after applying the correction for multiple comparisons using the Hochberg method where p > 0.9 (see figure 2).
The Cramer statistical test applied to estimate the effect size showed a value of 0.12, which shows a value with practical importance and presents a reduced phenomenon in our research.
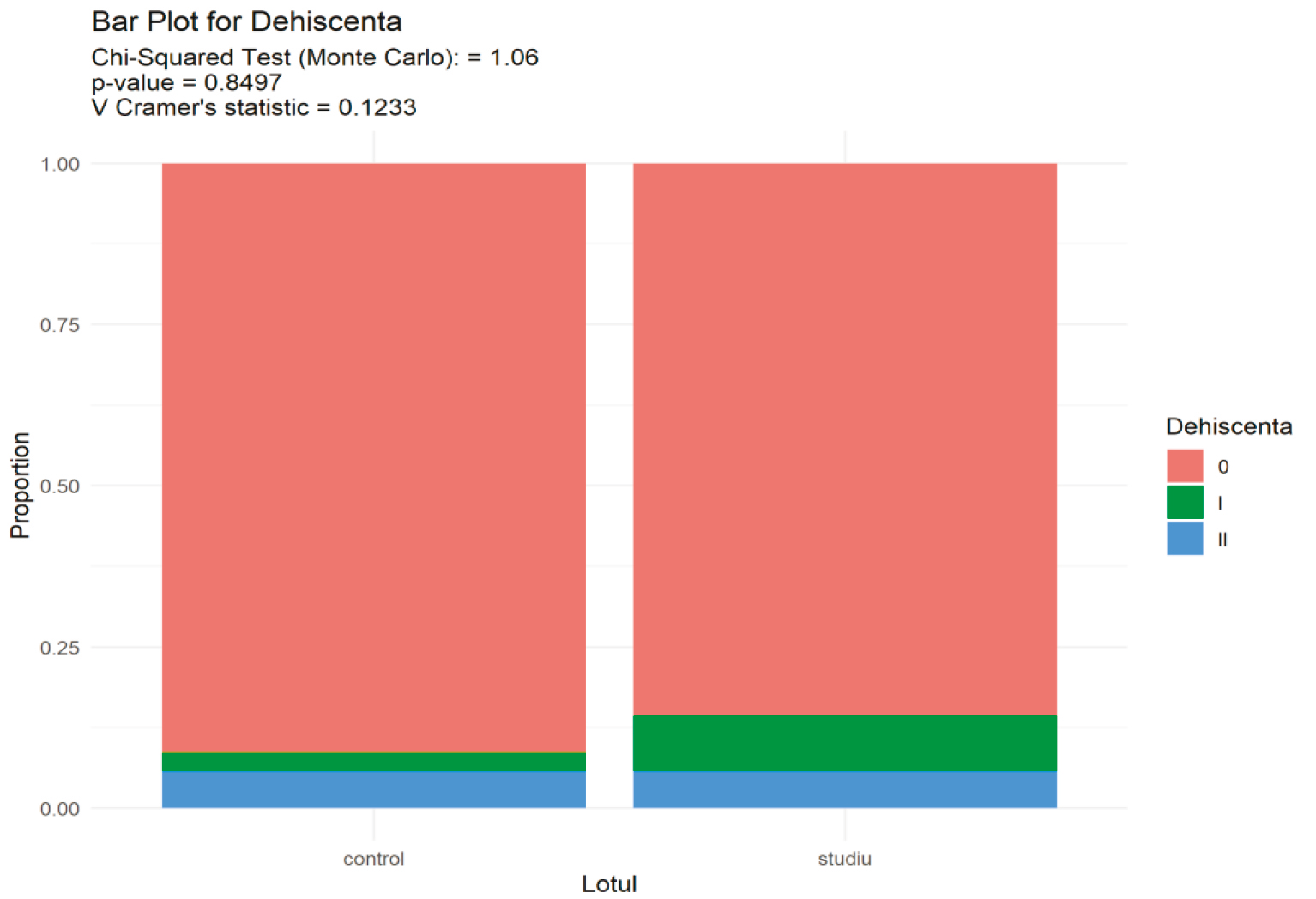 |
Fig. 2 Bar plot for the association values of flap formation technique and occurrence of dehiscence. Note: Applying the χ2 test (Monte Carlo variant) = 1.06, p=0.84, which means that there are no significant statistical differences, the result being unchanged even after applying the correction for multiple comparisons using the Hochberg method where p>0.9. Cramer statistical test - 0.12, which shows a value with no practical importance. |
Discussion
Recently, several techniques of flap formation have been proposed to achieve proper wound closure. Ronda et al. proposed a flap technique to achieve coronal displacement of the lingual flap in augmentation of the mandibular bone ridge in the posterior areas – a full-thickness lingual flap must be raised and partially detached from the mylohyoid muscle [16, 17].
Urban et al. describes this technique, dividing the surgical approach of the lingual flap into 3 different areas, as illustrated in figure 3 [18, 19]:
zone I: tunneling and elevation of the retromolar lampon.
zone II: separation of the flap with preservation of the mylohyoid muscle.
zone III: anterior periosteal release, semibont.
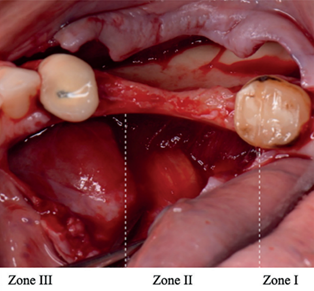 |
Fig. 3 The shape of the flap and the location of the surgical access areas.[20] |
This approach ensures adequate advancement of the lingual flap, which facilitates tension-free wound closure. Lingual flap mobilization is currently an imperial requirement in vertical bone augmentation of the mandible [19]. The lingual region of the mandible is considered a danger zone due to the anatomical elements located in the mandibulo-lingual groove, severe complications such as hemorrhages, hematomas, swellings have been reported in GBR implantation operations at the level of the mandible [20, 21]. The main cause is the specific anatomical area of the aforementioned groove, especially the sublingual artery which is a branch of the lingual artery, and which passes between the mylohyoid muscle and the genioglossus, it can give some small branches that supply the gum and can be easily damaged during flap preparation intervention [22]. Although the terminal branches of the lingual artery can be found in the lingual flap, the lingual artery is not at risk, provided that blunt dissection of the flap is performed. According to Urban et al. the most common complication is hemorrhage and is the result of trauma to the superficial periosteal terminal branches described above [19].
To achieve optimal wound adjustment and flap mobility, several flap processing techniques are described. Recently introduced flap advancement techniques may be superior to the classic periosteal release incision technique in large augmentation procedures. Periosteal release incision is reported to lead to several postoperative complications and may affect the blood supply to the flap. Buser et al. proposed the lateral flap incision technique in staged bone augmentation [23].
In the opinion of Porcaro et al., damage or excessive stress of the flap during surgery, a thin tissue biotype, and surgeon inexperience are factors that increase the risk of complications [21]. Maridati et al. specify that the flap must present a sufficiently large surgical field and allow complete coverage of the membrane with soft tissues and adequate vascularization. Flap repositioning should be tension free and not damaged during periosteal incisions to prevent soft tissue necrosis [24]. Fontana et al. mention that the most frequently used technique to allow the preservation of the blood supply to the flap is the incision of the flap through its entire thickness and with access to the upper part of the alveolar ridge [25].
Some surgeons like Kim et al. suggest making vertical release incisions to improve tissue mobility, while others report that this procedure reduces blood supply [2]. In many cases, the availability of soft tissues limits the potential for bone regeneration, and surgical augmentation of the soft tissue volume becomes necessary before the GBR procedure [26, 27]. The main disadvantages associated with the coronal advancement of the flap are the reduction of the depth of the vestibular fornix, the dislocation of the keratinized mucosal band and the mucogingival junction, and the generation of tissue tension. Reduction of the keratinized mucosal strip can compromise the aesthetic outcome of the future prosthetic construction and make oral hygiene procedures more difficult [28]. To reduce these risks, other flap formation techniques have been introduced by avoiding excessive sectioning of the periosteum, namely the periosteal release incision. The authors propose the following techniques for forming mucogingival flaps.
The double flap (DF) incision involves dividing the flap into two layers: an inner periosteal layer and an outer mucosal layer. The mucosal layer extends coronally and exceeds the height of the neighboring teeth to achieve primary closure, without traumatizing the periosteum, it is detached from the submucosa and the muscular insertion. It must be specified that the release of the periosteum starts from the level of the incision of the flap (the middle of the alveolar ridge) and is advanced towards the apex until the necessary release of the vestibular flap is achieved, technique illustrated in figure 4 [1, 9].
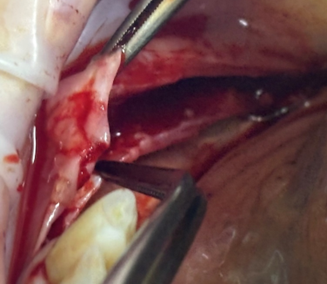 |
Fig. 4 Double flap (DF) technique. The buccal flap clearly reflects two separate layers, the periosteum separated bond and vestibular mucosa. |
The coronal advanced lingual flap (CALF) involves the detachment of the insertion of the mylohyoid muscle from the periosteum of the lingual flap, making it free to move coronally. It should be noted that the authors specify that the detachment of the muscle have to be done with a blunt instrument, atraumatic and without damaging the periosteum. Coronal advancement should be carried out at a height twice the height of the crowns of the neighboring teeth, technique illustrated in figure 5 [10, 16].
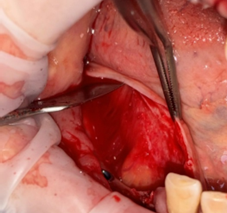 |
Fig. 5 Coronal advanced lingual flap (CALF), with its blunt release from the mylohyoid muscle. |
Modified Periosteal Releasing Incision (MPRI) introduced by Hur et al. in 2015 presents vestibular flap formation, and involves a superficial periosteal release incision similar to DF technique, but differs in that the level of incision and release of the periosteum begins at the level of the junction of the fixed and mobile mucosa and thus creates two segments: a segment of mucosa with coronal periosteum and the second, the apical periosteum which separated by abutment dissection with lateral extension according to the technique described in "DF" [28, 29]. The modified technique for performing the periosteal release flap is illustrated in figure 6 [9].
 |
Fig. 6 Modified Periosteal Releasing Incision" (MPRI) [9] |
According to the authors, in these sectional techniques the periosteum was involved less. They reported fewer complications compared to the classic periosteum release incision. A study comparing the types of flaps in GBR shows the following: according to Zazou et al. the coronal advanced lingual flap reported the highest advancement, the lowest post-intervention edema score and the lowest subsequent exposure [9]. Therefore, it is recommended that in large augmentation procedures in the posterior areas of the mandible, experienced manipulation of the lingual flap and knowledge of the anatomy are crucial to avoid injury to any vital structures in the given area. Noguera-Mutlló et al. in his work obtained that the detachment of the mylohyoid muscle from the lingual flap allows a significant increase in its extension, 2.5 times. Thus, this maneuver could be indicated in posterior mandibular bone augmentation procedures that require tension-free closure [20]. The results reported by Fugazzotto et al. show that with modified periosteal release technique and double vestibular flap formation achieved approximately 14.5 mm of its advancement, reporting the lowest mean pain score. This technique can be used in large bone augmentation operations with predictable results when used in patients with thick gingival tissue phenotype [30, 31].
Conclusion
The GBR technique is a complex method of bone augmentation, the success of which depends on several factors such as: protective membrane, flap formation, thread tension, as well as suture relaxation, which play key roles in guided bone growth. Strict adherence to the steps GBR minimizes the rate of complications, which are inevitable. In our research we demonstrated through statistical data that the occurrence of dehiscence has no correlation with the GBR technique used in our case, as the results were without statistical significance. Therefore, we can say with certainty that the small amount of dehiscence is mainly due to the shape and the formation disorder of the relaxed mucogingival flap, a fact also described in the specialized literature [30, 32]. Regardless of the chosen technique, it is important to consider the type and viability of the tissues used to cover these GBR elements.
Competing interests
None declared.
Authors’ contributions
VZ - conceptualization, investigation, methodology, writing - original draft, writing - review & editing, visualization, project administration, data curation, resources, DC - investigation; visualization, formal analysis. NC - investigation, project administration, validation, supervision, data curation. GC - supervision, data curation. All authors critically reviewed the work and approved the final version of the manuscript.
Acknowledgements and funding
The study had no external funding.
Patient consent
Obtained.
Ethics approval
The Research Ethics Committee of the Nicolae Testemițanu State University of Medicine and Pharmacy approved the study - Minutes no. 43 from 13.02.2020.
Authors’ ORCID IDs
Vasile Zugrav – https://orcid.org/0000-0002-7252-3498
Dumitru Chele – https://orcid.org/0000-0003-2231-8741
Nicolae Chele – https://orcid.org/0000-0001-8387-9062
Ghenadie Cucu – https://orcid.org/0000-0002-9551-0374
References
Thoma DS, Bienz SP, Figuero E, Jung RE, Sanz-Martín I. Efficacy of lateral bone augmentation performed simultaneously with dental implant placement: a systematic review and meta-analysis. J Clin Periodontol. 2019;46 Suppl 21:257-276. doi: 10.1111/jcpe.13050.
Kim Y, Kim TK, Leem DH. Clinical study of a flap advancement technique without vertical incision for guided bone regeneration. Int J Oral Maxillofac Implants. 2015;30(5):1113-8. doi: 10.11607/jomi.3586.
Wang HL, Boyapati L. “PASS” principles for predictable bone regeneration. Implant Dent. 2006;15(1):8-17. doi: 10.1097/01.id.0000204762.39826.0f.
Machtei EE. The effect of membrane exposure on the outcome of regenerative procedures in humans: a meta-analysis. J Periodontol. 2001;72(4):512-516.doi: 10.1902/jop.2001.72.4.512.
Retzepi M, Donos N. Guided Bone Regeneration: biological principle and therapeutic applications. Clin Oral Implants Res. 2010;21(6):567-576. doi: 10.1111/j.1600-0501.2010.01922.x.
Urban IA, Monje A, Wang HL, Lozada J, Gerber G, Baksa G. Mandibular regional anatomical landmarks and clinical implications for ridge augmentation. Int J Periodontics Restorative Dent. 2017;37(3):347-53. doi: 10.11607/prd.3199.
De Stavola L, Tunkel J. The role played by a suspended external-internal suture in reducing marginal flap tension after bone reconstruction: a clinical prospective cohort study in the maxilla. Int J Oral Maxillofac Implants. 2014;29(4):921-926. doi: 10.11607/jomi.3370.
Burkhardt R, Lang NP. Role of flap tension in primary wound closure of mucoperiosteal flaps: a prospective cohort study. Clin Oral Implants Res. 2010;21(1):50-54.doi: 10.1111/j.1600-0501.2009.01829.x.
Zazou N, Diab N, Bahaa S, El Arab AE, Aziz OA, El Nahass H. Clinical comparison of different flap advancement techniques to periosteal releasing incision in guided bone regeneration: a randomized controlled trial. Clin Implant Dent Relat Res. 2021;23(1):107-116. doi: 10.1111/cid.12960.
Canullo L, Tronchi M, Kawakami S, Iida T, Signorini L, Mordini L. Horizontal bone augmentation in the anterior esthetic area of the maxilla using a flap design adapted from mucogingival surgery in association with PLA membrane and β-TCP. Int J Periodontics Restorative Dent. 2019;39(2):195-201. doi: 10.11607/prd.3894.
Gallo P, Díaz-Báez D. Management of 80 complications in vertical and horizontal ridge augmentation with nonresorbable membrane (d-PTFE): a cross-sectional study. Int J Oral Maxillofac Implants. 2019;34(4):927-935. doi: 10.11607/jomi.7214.
Urban IA, Saleh MHA, Ravidà A, Forster A, Wang HL, Barath Z. Vertical bone augmentation utilizing a titanium‐reinforced PTFE mesh: a multi‐variate analysis of influencing factors. Clin Oral Implants Res. 2021;32(7):828-839. doi: 10.1111/clr.13755.
Steigmann M, Salama M, Wang HL. Periosteal pocket flap for horizontal bone regeneration: a case series. Int J Periodontics Restorative Dent. 2012;32(3):311-20.
Fontana F, Maschera E, Rocchietta I, Simion M. Clinical classification of complications in guided bone regeneration procedures by means of a nonresorbable membrane. Int J Periodontics Restorative Dent. 2011;31(3):265-73.
Al-Ardah AJ, AlHelal A, Proussaefs P, AlBader B, AlHumaidan AA, Lozada J. Managing titanium mesh exposure with partial removal of the exposed site: a case series study. J Oral Implantol. 2017;43(6):482-490.
Ronda M, Stacchi C. A novel approach for the coronal advancement of the buccal flap. Int J Periodontics Restorative Dent. 2015;35(6):795-801. doi: 10.11607/prd.2232.
Ronda M, Stacchi C. Management of a coronally advanced lingual flap in regenerative osseous surgery: A case series introducing a novel technique. Int J Periodontics Restorative Dent. 2011;31(5):505-513.
Urban IA, Monje A, Lozada J, Wang HL. Principles for vertical ridge augmentation in the atrophic posterior mandible: a technical review. Int J Periodontics Restorative Dent. 2017;37(5):639-45. doi: 10.11607/prd.3200.
Urban I, Traxler H, Romero-Bustillos M, Farkasdi S, Bartee B, Baksa G, et al. Effectiveness of two different lingual flap advancing techniques for vertical bone augmentation in the posterior mandible: a comparative, split-mouth cadaver study. Int J Periodontics Restorative Dent. 2018;38(1):35-40. doi: 10.11607/prd.3227.
Noguera-Mutlló C, Traboulsi-Garet B, Camps-Font O, Manzanares-Céspedes MC, Figueiredo R, Valmaseda-Castellón E. Comparison of two different lingual flap advancement techniques and vascular structure identification: a human cadaver study. Med Oral Patol Oral Cir Bucal. 2022;27(6):e532-e538. doi: 10.4317/medoral.25451.
Porcaro G, Busa A, Bianco E, Caccianiga G, Maddalone M. Use of a partial-thickness flap for guided bone regeneration in the upper jaw. J Contemp Dent Pract. 2017;18(12):1117-1121. doi: 10.5005/jp-journals-10024-2186.
Sîrbu D, Mostovei M, Strîşca S, Popovici V, Mighic A, Mighic V. Particularităţile planificării și tratamentului protetic în reabilitarea pacienţilor edentaţi cu inserarea angulată a implantelor [Planing and prosthetic treatment particularities in rehabilitation of edentulous patients with tilted implant placement]. Med Stomatol (Chisinau). 2017;(3):54-60. Romanian.
Buser D, editor. 20 years of guided bone regeneration in implant dentistry. 2nd ed. Chicago: Quintessence Pub. Co; 2009. 261 p.
Maridati PC, Cremonesi S, Fontana F, Cicciù M, Maiorana C. Management of d-PTFE membrane exposure for having final clinical success. J Oral Implantol. 2016;42(3):289-291. doi: 10.1563/aaid-joi-D-15-00074.
Fontana F, Grossi GB, Fimanò M, Maiorana C. Osseointegrated implants in vertical ridge augmentation with a nonresorb able membrane: a retrospective study of 75 implants with 1 to 6 years of follow-up. Int J Periodontics Restorative Dent. 2015;35(1):29-39. doi: 10.11607/prd.2136.
Pietrokovski J, Massler M. Alveolar ridge resorption following tooth extraction. J Prosthet Dent. 1967;17(1):21-27.doi: 10.1016/0022-3913(67)90046-7.
Sîrbu D, Topalo V, Rusnac C, Strișca S, Suharschi I, Mighic A, Ghețiu A, Căldărari S. A-PRF o nouă direcţie a regenerării tisulare în chirurgia dento-alveolară [Tissue engineering with Platelet-Rich Fibrin in oral region]. Med Stomatol (Chisinau). 2016;(1-2):16-23. Romanian.
Hur Y, Tsukiyama T, Yoon T-H, Griffin T. Double flap incision design for guided bone regeneration: a novel technique and clinical considerations. J Periodontol. 2010;81(6): 945-952. doi: 10.1902/jop.2010.090685.
Hur Y, Bui MN, Griffin TJ, Ogata Y. Modified periosteal releasing incision for flap advancement: a practical technique for tensionless closure. Clin Adv Periodontics. 2014;5(4):229-234. doi: 10.1902/cap.2014.140009
Plonka AB, Sheridan RA, Wang Hom-Lay. Flap designs for flap advancement during implant therapy: a systematic review. J Oral Implantol. 2017;26(1):145-152.
Fugazzotto PA. Maintaining primary closure after guided bone regeneration procedures: Introduction of a new flap design and preliminary results. J Periodontol. 2006;77(8):1452-1457. doi: 10.1902/jop.2006.050392.
Zugrav V, Chele N. Noi abordări în tratamentul dehiscențelor gingivale în Regenerarea Osoasă Ghidată. Sinteză literară [New approaches in the treatment of gingival dehiscences in Guided Bone Regeneration. A systematic literature review]. Med Stomatol. 2023;(4):6-15. https://doi.org/10.53530/1857-1328.23.4.01. Romanian.

