Introduction
The majority of plasma cell neoplasms and B-cell lymphoproliferative processes are characterized by clonal immunoglobulin gene rearrangement and expression of cytoplasmic or surface immunoglobulin with kappa or lambda light chain restriction.
Heavy immunoglobulin gene rearrangement can be demonstrated by polymerase chain reaction on formalin-fixed, paraffin-embedded (FFPE) tissues although the procedure is time-consuming, expensive, and has a variable (65-73%) sensitivity [1, 2]. An alternative way to prove clonality is immunological detection of light chain restriction, which is largely performed on cells in suspension by flow cytometric immunophenotyping or direct immunofluorescence microscopy (DIFM) on frozen tissues. Since fresh tissue is not always available, immunohistochemical (IHC) staining for kappa and lambda light chains is often performed on FFPE tissues [3-6]. Commercially available polyclonal antibodies to human kappa and lambda light chains have high background and false negative rates, which makes it difficult to prove clonality. In addition, polyclonal antibodies and the majority of monoclonal antibodies (mAbs) available on the market require digestion of tissue sections with proteolytic enzymes, which may alter tissue morphology and give false negative results due to protein over-digestion, particularly if specimens are poorly fixed. We report here a series of new mAbs to constant regions of human kappa and lambda light chains suitable for IHC staining and DIFM of FFPE tissues. Epitope enhancement was performed at a constant and uniform temperature in a water bath and does not require the use of proteolytic enzymes [7].
Materials and methods
Monoclonal antibody production
Monoclonal antibodies directed to the constant region of human immunoglobulin kappa and lambda light chains were produced by immunization of BALB/c mice with purified human immunoglobulin light chains (Bence Jones protein provided by Dr. A. Solomon from University of Tennessee, Knoxville, and from Bethyl Laboratories, Inc., Montgomery, TX) in the presence or absence of CpG oligodeoxynucleotide 1826 (InvivoGen, San Diego, CA). After final boost, the spleens were removed and splenocytes were fused with NS1 myeloma cells, as previously described [8]. Primary screening of the hybridoma cell lines was performed by a modified enzyme-linked immunosorbent assay (ELISA). In brief, the 96-well ELISA plates were coated with 0.2 g/well purified human kappa or lambda light chains fixed with 10% formalin. Hybridoma cell lines secreting antibodies that would react only with free kappa and lambda light chains were then excluded by ELISA using plates coated with purified human IgG (Sigma, St. Louis, MO). Positive hybridoma populations were selected by immunohistochemical staining of FFPE tissue without antigen retrieval. For this purpose, we used tissue microarray constructs containing multiple 3 mm tissue cylinders retrieved from donor blocks of plasma cell neoplasms with known immunophenotype. Hybridoma cell lines staining plasma cell neoplasms on FFPE tissues without antigen retrieval were subcloned and used for production of ascitic fluids in mice.
Antibody purification and labeling
Monoclonal antibodies from ascites were purified by ion exchange chromatography using DEAE Sepharose (Sigma-Aldrich, St. Louis, MO). Purified antibodies were labeled with Alexa Fluor 488 or R-PE by using Alexa Fluor 488 Protein Labeling Kit (Molecular Probes, Eugene, OR) and AnaTag R-Phycoerythrin Protein Labeling Kit (AnaSpec, Inc., San Jose, CA), respectively.
Immunohistochemical staining and direct immunofluorescence microscopy
4-um thick sections of FFPE tissues mounted onto plus slides were deparaffinized in xylene, rehydrated in graded alcohols, and endogenous peroxidase activity was blocked with 1% hydrogen peroxide in distilled water. The water bath (Isotemp 205, Fisher Scientific) containing the staining dish with 250 ml of epitope enhancement buffer was pre-heated to 97oC. The slides were immersed in the staining dish and incubated for 45 minutes. The reduction of high background staining on certain types of tissues (“bloody specimens”) was achieved by treating the tissue sections for 10 min with ReBlot Plus mild antibody striping solution (Chemicon, Temecula, CA) immediately after the epitope enhancement procedure. The sections were then washed in buffer solution (DakoCytomation, Carpinteria, CA) for three minutes and incubated with 30 ng/ml mAbs to kappa or lambda light chains for 30 minutes. After washing twice for 5 minutes the bound primary antibodies were detected with EnVision+ System-HRP from DakoCytomation (Carpinteria, CA) with diaminobenzidine chromogen substrate. For DIFM studies, tissue sections after epitope enhancement were incubated for 30 minutes with 70 ng/ml Alexa Fluor 488 and/or R-PE labeled mAbs to kappa and lambda light chains and examined under fluorescence microscope.
Results and Discussion
Production and characterization of mAbs to human immunoglobulin constant- region light chain isotypes.
Our first attempt to generate mAbs by immunizing mice with a mixture of kappa and lambda light chains in the presence of CpG oligodeoxynucleotide 1826 (CpG ODN) as an adjuvant [9] failed to detect hybridoma cell lines secreting antibodies to kappa protein. The great majority of hybridoma populations secreted antibodies to lambda protein. In contrast, when mice were immunized with purified kappa light chains without CpG ODN a large proportion of hybridoma cell lines secreted antibodies to kappa protein. Since the main goal of this study was to generate mAbs to antigenic epitopes of kappa and lambda light chains either unaffected or minimally affected by formalin fixation, we utilized a novel strategy for screening and selection of antibody-producing hybridoma populations.
Primary screening was performed by ELISA in plates coated with kappa and lambda light chains that were fixed with 10% formalin after coating the wells. The rationale for formalin fixation was to select hybridoma populations secreting antibodies to antigenic epitopes either unaffected or minimally affected by cross-linkage of formaldehyde groups. After the first screen, approximately 120 hybridomas were selected from each of the kappa or lambda fusions. The second screen was performed to exclude hybridoma populations that secreted antibodies that would react with variable-region subgroups, free light chains not associated with immunoglobulin molecules, and antibodies cross-reacting with both kappa and lambda proteins. This goal was achieved by testing antibody reactivity with kappa and lambda light chains from patients with different variable subgroups and purified human polyclonal IgG. As a result, 41 hybridoma populations secreting antibodies to kappa (21) and lambda (20) light chains were selected for immunohistochemical staining of FFPE tissues without antigen retrieval.
IHC was performed on sections of tissue microarray constructs containing nine 3 mm tissue cylinders retrieved from donor blocks of plasma cell neoplasms with known immunophenotype. By IHC we selected eight hybridoma populations that produced highly specific mAbs to kappa (4 populations) and lambda light chains (4 populations) for subcloning and ascites production in mice. All of the mouse mAbs were either of IgG1, 2a or 2b isotypes.
Immunohistochemical staining of FFPE tissues
For all IHC and DIFM studies the culture media, the mixture of purified and labeled antibodies to kappa (4 clones) or lambda (4 clones), were diluted to a final concentration of approximately 30 ng/ml (IHC) or 70 ng/ml (DIFM). Staining the sections of FFPE normal tonsil without epitope enhancement detects primarily plasma cells located in interfollicular areas and submucosa (Fig. 1A). When a heat-based epitope enhancement procedure is used, the detection of plasma cells and large B-cells (transformed cells) in interfollicular areas is markedly enhanced (Fig. 1B). Moreover, a subset of large centroblasts located in germinal centers is also detected. The staining of small mature lymphocytes in mantle zones or centrocytes in germinal centers is equivocal, probably due to a low density of B-cell receptors on these cells.
Immunoglobulin proteins are widely present in all tissues and interstitial fluid, which inevitably give high background staining and make the interpretation in some instances very difficult. In the tonsil, immunoglobulins are also present on epithelial cell surfaces and are readily revealed by IHC staining with anti-kappa or anti-lambda antibodies. Since the immunoglobulins present in the interstitial fluid and on epithelial surface are not an integral part of cell membranes, we hypothesized that these proteins may be partially removed by pre-treating the tissue sections with stripping agents. Fig. 1C shows a normal tonsil stained with mAbs to kappa and lambda light chains, which demonstrate high background staining of the mucosal surface. Pretreatment of the section for 10 min with ReBlot Plus mild antibody striping solution significantly reduced the background staining of mucosal lining without affecting the staining of plasma cells and large B-cells (Fig. 1D). However, it appears that this pretreatment attenuates staining of B-cell in the mantle zones.
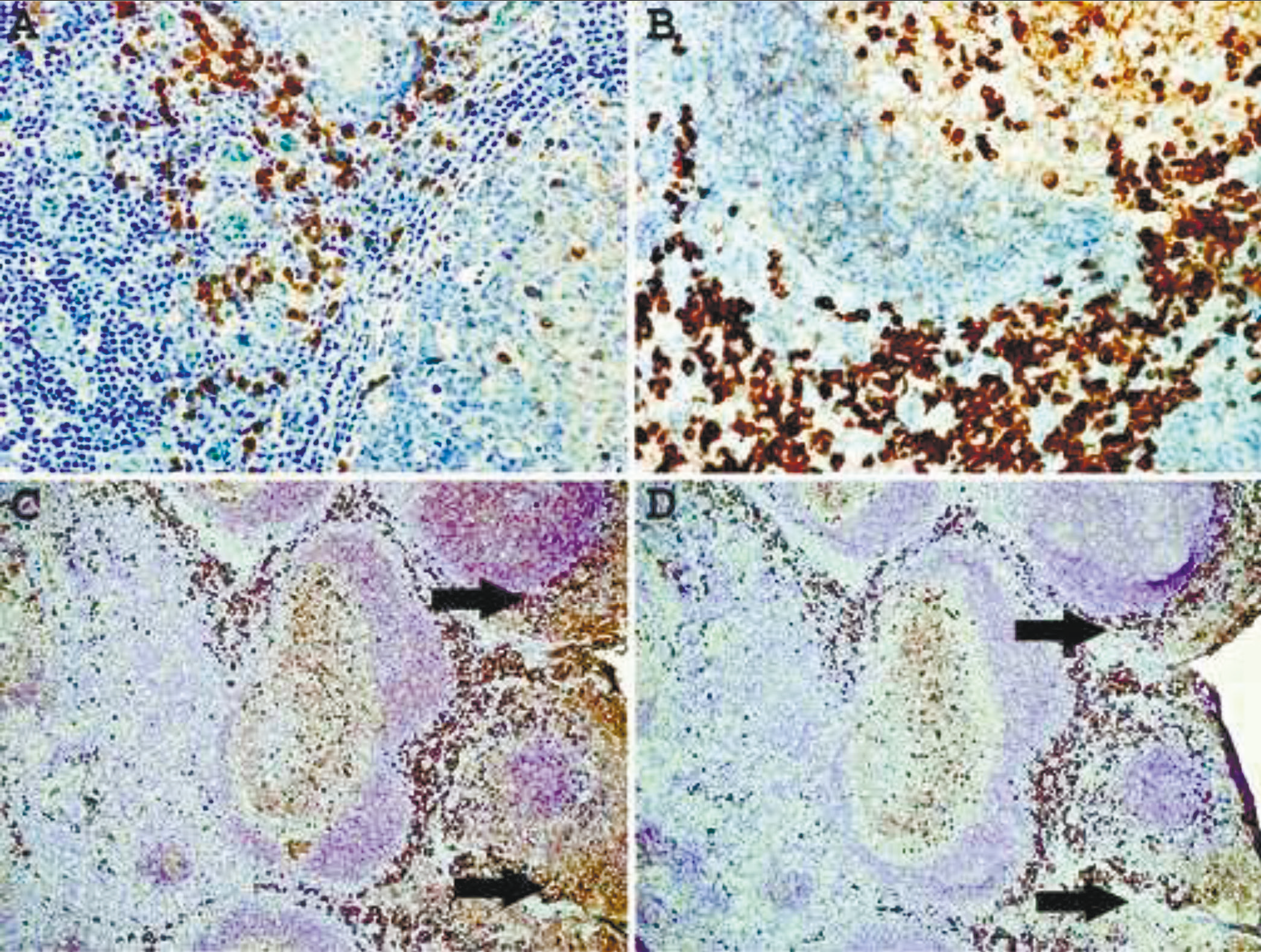 |
Fig. 1 Immunohistochemical staining of formalin-fixed, paraffin-embedded normal tonsil with a mixture of mAbs to kappa and lambda light chains. A – no epitope enhancement; B – heat-based epitope enhancement. Original magnification 40X. C - untreated and treated (D)sections of thetonsil for 10 min withReBlot Plus mildantibody striping solution after epitope enhancement. Arrows indicate high background staining. Note attenuation of the mantlezone staining afterstriping procedure. Original magnification 10X. |
Staining of plasma cell neoplasms with our mAbs usually does not require epitope enhancement (Fig.2 A, B) although after epitope enhancement the staining is more uniform and intense with minimal increase in background staining (Fig. 2 C-F). Clonality of some B-cell neoplasms, in particular lymphomas composed of large cells with abundant cytoplasm or plasmacytic differentiation can also be readily demonstrated by IHC staining. As can be seen from Fig. 3 A, B, lambda light chain restriction is appreciated in a diffuse large B-cell lymphoma (DLBCL) with plasmablastic features. An example of large B-cell lymphoma with anaplastic features showing lambda light chain restriction is demonstrated in Fig. C, D. A case of angioimmunoblastic T-cell lymphoma associated with monoclonal expansion of large B-cells (immunoblasts) is shown in Fig. 3 E, F. Flow cytometry immunophenotyping on cell suspension from this lymph node stained with our mAbs conjugated with Alexa Fluor 488 and R-PE revealed a kappa light chain restricted lymphoid population with a kappa to lambda ratio of 8:1. Approximately the same kappa to lambda ratio is demonstrated on IHC staining with mAbs (Fig. 3E, F).
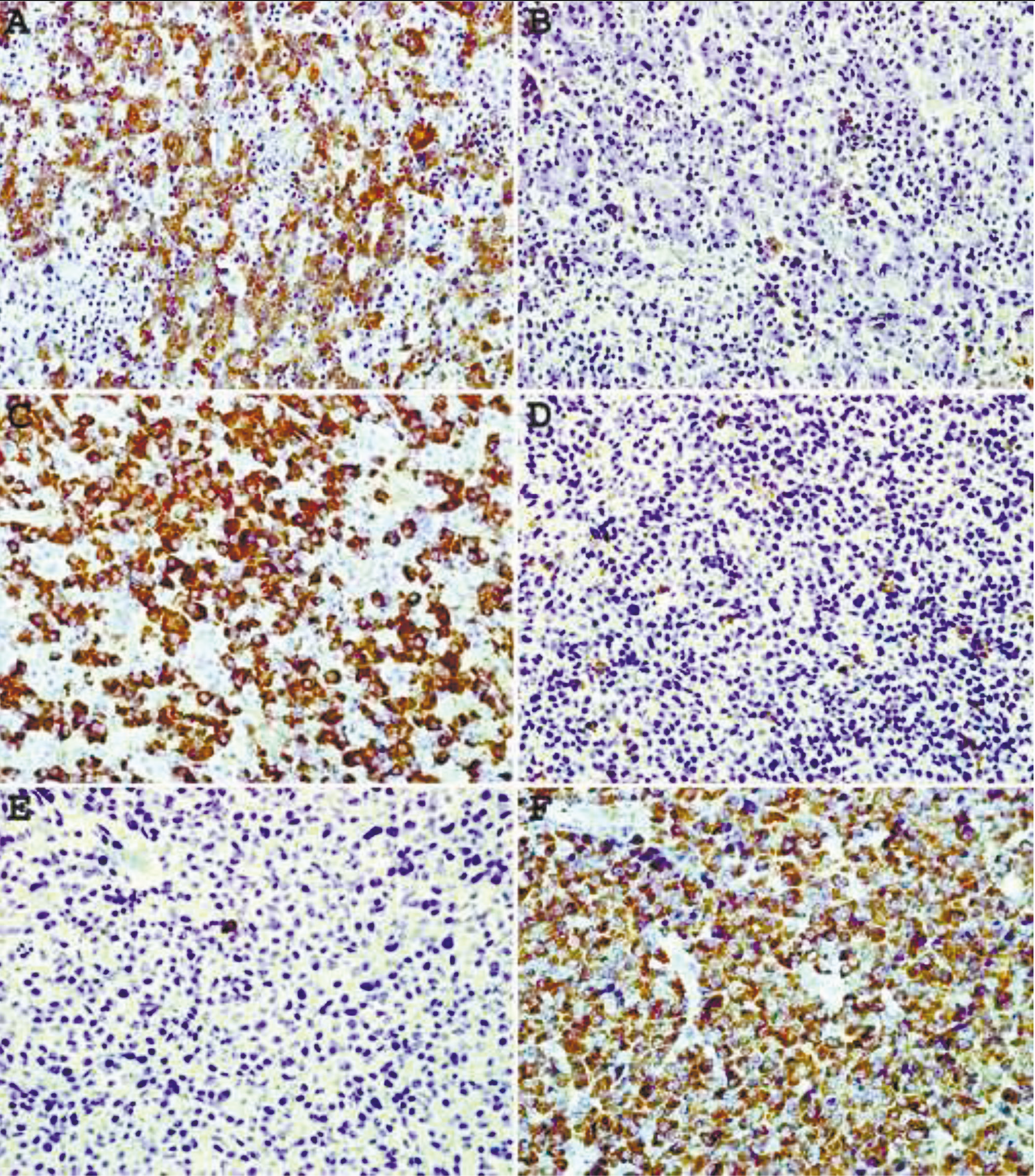 |
Fig. 2 Immunohistochemical staining of plasma cell neoplasm with mAbs to kappa (A, C, E) and lambda (B, D, F) lightchains. A, B – no epitope enhancement. C-F – heat- based epitope enhancement. Original magnification 40X. |
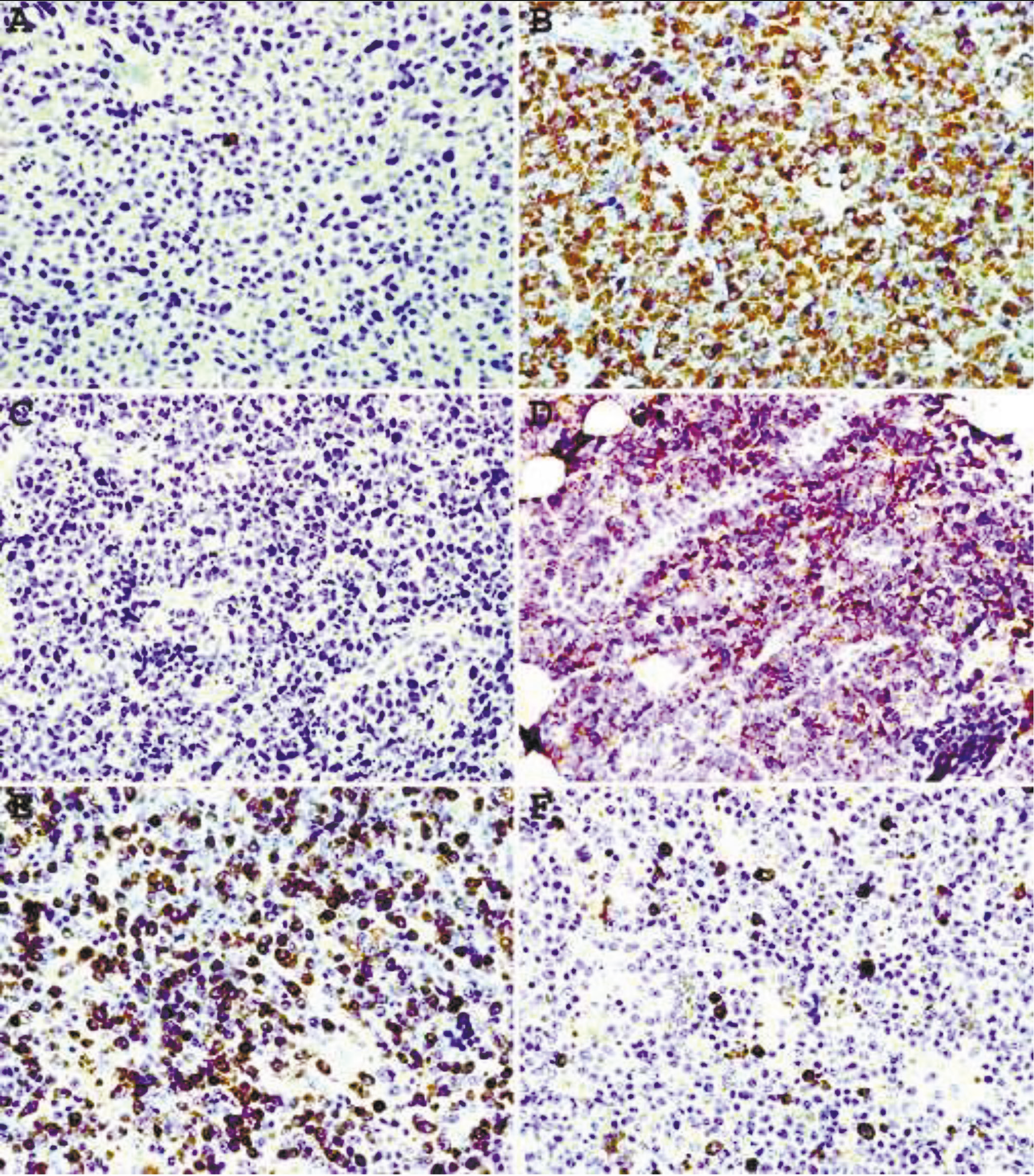 |
Fig. 3Immunohistochemical staining withepitope enhancement of a diffuse large B-cell lymphoma with plasmablastic features (A, B), highly pleomorphic plasma cell myeloma (C, D), and angioimmunoblastic T-celllymphoma associated with clonal immunoblastic proliferation (E, F). A, C, E – mAbs to kappa light chain; B, D, F – mAbs to lambda light chain. Original magnification 40X. |
Direct immunofluorescence microscopy of FFPE tissues
In a large portion of diffuse large B-cell lymphomas and in a subset of plasma cell neoplasm no immunoglobulin expression can be demonstrated by IHC. This may be due to a high background or lack of surface immunoglobulin on a large subset of diffuse large B-cell lymphomas [10, 11]. We labeled antibodies to kappa and lambda light chains with Alexa Fluor 488 and R-PE, respectively, and used them for DIFM of FFPE tissues.
Staining a normal tonsil with anti-kappa antibodies labeled with Alexa Fluor 488 shows a lower background staining intensity in surface epithelium (Fig. 4A) compared to IHC staining (Fig. 2A). Combining anti-kappa and anti-lambda antibodies clearly illustrates the polyclonal nature of plasma cells and large B-cells in reactive lymphoid hyperplasia on the same section of the tonsil (Fig. 4B). This antibody cocktail was also used to demonstrate kappa (Fig. 4C) or lambda (Fig.4D) light chain restriction in two cases of DLBCL.
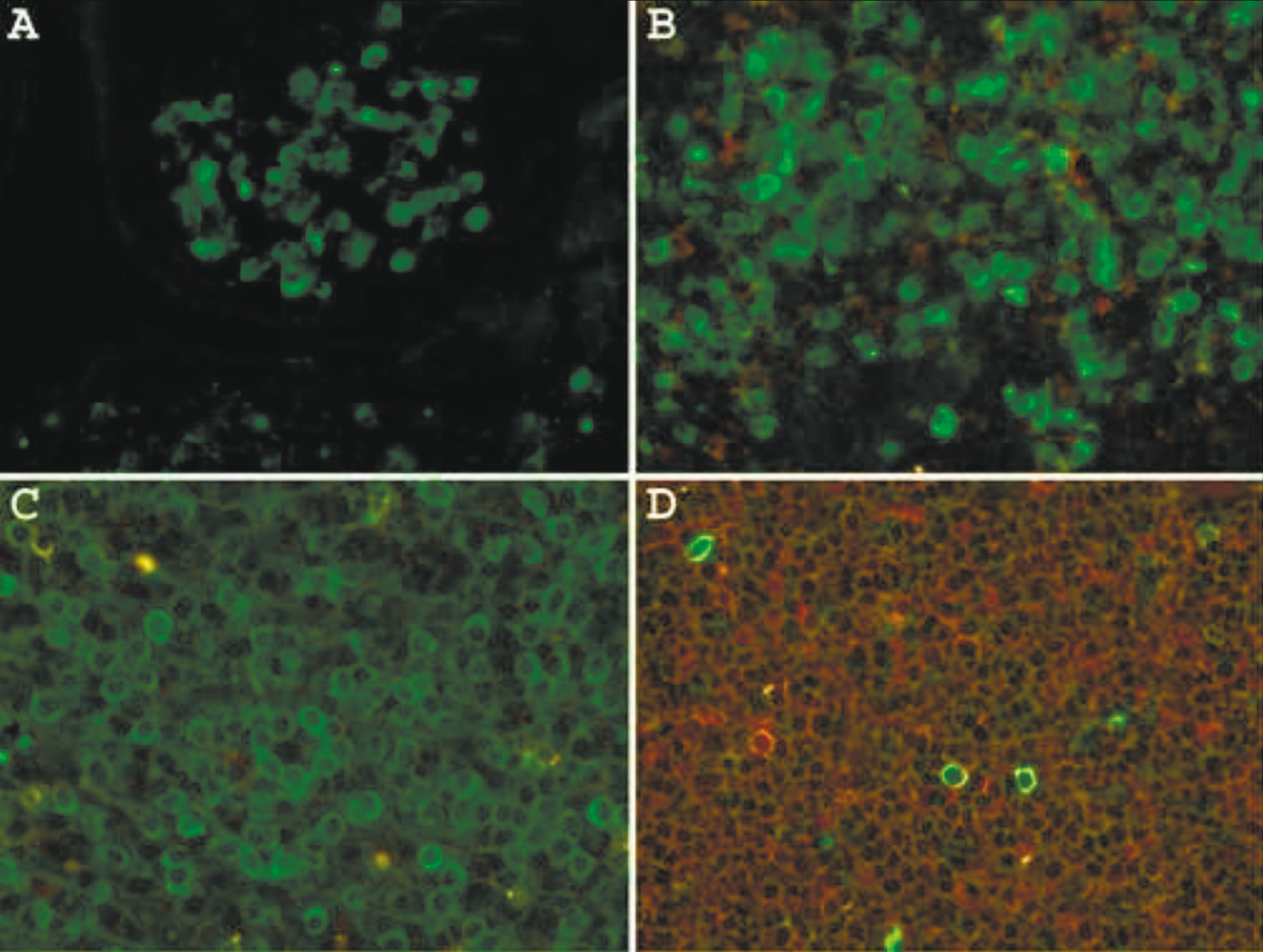 |
Fig. 4 Direct immunofluorescence microscopy on formalin-fixed, paraffin-embedded tissue using a heat-based epitope enhancement procedure. Green fluorescence represents mAbs to kappa light chain labeled with Alexa Fluor 488 and in red fluorescence are mAbs to lambda light chain labeled with R-Phycoerythrin. A. Normal tonsil stained with anti-kappa. B. Normal tonsilstained with anti-kappa and anti-lambda. C. Diffuse large B-cell lymphoma kappa light chainrestricted; D. Diffuse large B-cell lymphoma, lambda light chain restricted. Original magnification 60X. |
Comparison of monoclonal antibodies with commercially available polyclonal antibodies to kappa and lambda light chains
Twenty-two cases of known plasma cell neoplasms were selected from the files of the Department of Pathology, University of Iowa Hospitals and Clinics. All cases were stained at the time of diagnosis with commercial polyclonal antibodies after proteolytic digestion of deparaffinized sections with Protease K. Seven cases were kappa and 5 cases were lambda-restricted, respectively; 4 cases were “indeterminate” or “equivocal”; 6 cases were negative; and one case was very weakly positive for lambda light chain (Table 1). Four cases with equivocal staining had a high background when stained with polyclonal antibodies to kappa and lambda light chains, which made the interpretation very difficult. Interestingly, all equivocal and 5/6 negative cases showed lambda light chain restriction by IHC staining with or without antigen retrieval when our mAbs were utilized. Serum protein immunofixation electrophoresis (SIFE) on 8/9 of these equivocal and negative cases showed the presence of a monoclonal protein, either IgG- (5 cases) or IgA- (3 cases). For case 19 the SIFE results were unknown. Case 12 stained very weakly with both polyclonal and monoclonal antibodies. A case of CD30-positive plasmablastic lymphoma (case 23) was completely negative with polyclonal and monoclonal antibodies to both light chain isotypes. This data suggests that polyclonal antibodies to lambda light chains have a high false negative rate (5 out of 15 cases) compared to anti-kappa antibodies.
Table 1. Comparative immunohistochemical staining of FFPE tissues of plasma cell neoplasms with commercial polyclonal antibodies and monoclonal antibodies to kappa and lambda light chains | ||||||
Patients | PA with EE | mAbs withEE | mAbs without EE | |||
| anti-κ | anti-λ | anti-κ | anti-λ | anti-κ | anti-λ |
1 | - | + | - | + | ND | ND |
2 | - | + | - | + | ND | ND |
3 | - | - | - | + | ND | ND |
4 | + | - | + | - | ND | ND |
5 | + | - | + | - | ND | ND |
6 | + | - | + | - | ND | ND |
7 | Ind | Ind | - | + | ND | ND |
8 | - | - | - | + | ND | ND |
9 | - | - | - | + | ND | ND |
10 | - | - | - | + | - | + |
11 | - | - | - | + | - | + |
12 | - | +/- | - | +/- | - | +/- |
13 | - | + | - | + | - | + |
14 | + | - | + | - | ND | ND |
15 | + | - | + | - | + | - |
16 | + | - | + | - | + | - |
17 | - | + | - | + | - | + |
18 | Ind | Ind | - | + | - | + |
19 | Ind | Ind | - | + | - | + |
20 | + | - | + | - | ND | ND |
21 | Ind | Ind | - | + | - | + |
22 | - | + | - | + | ND | ND |
23 | - | - | - | - | ND | ND |
Note: FFPE – formalin-fixed, paraffin-embedded, PA – polyclonal antibodies; EE- epitope enhancement; Ind – indeterminate; ND- not done; mAbs – monoclonal antibodies | ||||||
In conclusion, our new mAbs have lower false negative rates and lower background staining compared to commercial polyclonal antibodies. In some specimens (“bloody tissues”) the high background staining may be significantly reduced by treating the sections with ReBlot Plus mild antibody striping solution. It also appears that DIFM is more sensitive and has a lower background staining compared to IHC and may be used for immunophenotyping of large B-cell neoplasms.
Competing interests
None declared.
Funding
No external funding.
Acknowledgment
The authors thank Dr. Alan Solomon (University of Tennessee, Knoxville), for the kind provision of the purified Bence Jones proteins used in our study for mice immunization and screening ELISA, Dr. Oscar Rokhlin for guiding the hybridoma aspect of the project, and Drs. John Kemp and Barry De Young for helpful critical comments and suggestions.
Authors’ ORCID IDs
Sergei I. Syrbu – https://orcid.org/0000-0003-4620-7923
Michael B. Cohen – https://orcid.org/0000-0002-6111-025X
References
1. Camilleri-Broet S, Devez S, Tissier F, et al. Quality control and sensitivity of polymerase chain reaction techniques for the assessment of immunoglobulin heavy chain gene rearrangements from fixed- and paraffin-embedded samples. Ann Diagn Pathol. 2000;4(2):71-6. doi: 10.1016/s1092-9134(00)90014-5.
2. Inghirami G, Szabolcs MJ, Corradini P, et al. Detection of immunoglobulin gene rearrangement of B cell non-Hodgkin lymphomas and leukemias in fresh, unfixed and formalin-fixed, paraffin-embedded tissue by polymerase chain reaction. Lab Invest. 1993;68(6):746-57.
3. Ashton-Key M, Jessup E, Isaacson PG. Immunoglobulin light chain staining in paraffin-embedded tissue using a heat mediated epitope retrieval method. Histopathology. 1996;29(6):525-31. doi: 10.1046/j.1365-2559.1996.d01-534.x.
4. Leong AS, Yin H, Hafajee Z. Patterns of immunoglobulin staining in paraffin- embedded malignant lymphomas. Appl Immunohistochem Mol Morphol. 2002;10(2):110-4. doi: 10.1097/00129039-200206000-00003.
5. Marshal-Taylor CE, Cartun RW, Mandich D, et al. Immunohistochemical detection of immunoglobulin light chain expression in B-cell non-Hodgkin lymphomas using formalin-fixed, paraffin-embedded tissues and a heat-induced epitope retrieval technique. Appl Immunohistochem Mol Morphol. 2002;10(3):258-62. doi: 10.1097/00129039-200209000-00013.
6. Merz H, Rickers O, Orscheschek K, et al. Constant detection of surface and cytoplasmic immunoglobulin heavy and light chain expression in formalin-fixed and paraffin-embedded material. J Pathol. 1993;170(3):257-64. doi: 10.1002/path.1711700307.
7. Syrbu SI, Cohen MB. An enhanced antigen retrieval procedure for immunohistochemical staining of formalin-fixed, paraffin-embedded tissue. In: Kalyuzhny A, editor. Signal transduction immunohistochemistry: Methods and protocols. New York: Humana Press; 2011. p. 101-110. (Methods in Molecular Biology; vol. 717).
8. Davidson RL, Gerald PS. Improved techniques for the induction of mammalian cell hybridization by polyethylene glycol. Somatic Cell Genet. 1976;2(2):165-76. doi: 10.1007/BF01542629.
9. Weiner GJ, Liu H-M, Wooldrich JE, et al. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc Natl Acad Sci USA. 1997;94(20):10833-37. doi: 10.1073/pnas.94.20.10833.
10. Loddenkemper C, Anagnostopoulos I, Hummel M, Jöhrens-Leder K, Foss HD, Jundt F, Wirth T, Dörken B, Stein H. Differential Emu enhancer activity and expression of BOB.1/OBF.1, Oct2, PU.1, and immunoglobulin in reactive B-cell populations, B-cell non-Hodgkin lymphomas, and Hodgkin lymphomas. J Pathol. 2004 Jan;202(1):60-9. doi: 10.1002/path.1485.
11. Pileri SA, Gaidano G, Zinzani PL, et al. Primary mediastainal B-cell lymphoma: high frequency of BCL-6 mutations and consistent expression of the transcription factors OCT-2, BOB.1, and PU.1 in the absence of immunoglobulins. Am J Pathol. 2003;162(1):243-53. doi: 10.1016/s0002-9440(10)63815-1.

