Introduction
One of the leading disorders among females’ population is considered to be breast cancer. It becomes younger in time and brings a serious threat to women’s life as well as physical and mental health.
The pathogenesis of breast cancer is still unclear, which leads to poor outcomes of prevention and treatment of this disease [1]. In the past decades, the most of investigations were focused primarily on tumor cells. However, it is well known, that tumors are composed of parenchyma and stroma, two distinct but cooperative components that can influence malignant cells growth and spread. Many recent studies show that the tumor microenvironment plays an important role in tumor initiation, therefore it can be a fertile soil for disease progression [2]. The tumor microenvironment is made of cells and extracellular matrix, where the cellular component includes different types of cells, such as myofibroblasts, fibroblasts, myoepithelial cells, blood and lymphatic endothelial cells (and their precursors), pericytes, inflammatory cells and mesenchymal stem cells [3, 4]. Epithelial-mesenchymal correlations are crucial for normal mammary gland development and therefore they can play a critical role for breast cancer development and progression. The peritumoral environment interacts with parenchymal cancerous cells and can create favorable condition for tumor grow and invasion.
Cell-surface glycoprotein CD34 represents a glycosylated protein expressed by hematopoietic stem/progenitor cells, endothelial and mesenchymal cells at different sites, including breast [5]. Its function consists in the modulation of cell adhesion and signal transduction. This receptor is particularly sensitive to tumor angiogenesis, because it can clearly represent the state of neovascularization during the growth and progression of tumor [6]. Furthermore, Kaplan-Meier analysis showed that the survival time of patients with high CD34 expression was significantly shorter than that of patients with low CD34 expression. In another words, a high level of CD34 expression may be a potential indicator of a poor prognosis. The new made vessels provide nutrients for tumor cells and remove metabolic waste, thus promoting the growth rate of tumor [7].
Another raising life- and health-threatening chronic disease is diabetes mellitus (DM) type 2. When associated with cancer, it can worsen tumors’ evolution and increase the mortality risk. Both of these disorders are the major causes of death worldwide [8]. However, there are limited evidences supporting this correlation that seems to be very complex and it needs further epidemiological, morphological, and molecular investigation. Insufficient care for either diabetes mellitus or breast cancer can affect the survival [9]. The wandering stuff is to investigate the impact of preexisting diabetes on the histopathological structure of breast cancer. It can give a reasonable explanation about breast cancer behavior on the diabetes mellitus background.
Thus, until now there are no evidences about how associated diabetes can influence the content of CD34+ vessels inside of breast tumor and in the peritumoral area. The aim of this study was to clarify how diabetes mellitus, which affects many tissues, can influence the breast cancer environment. Therefore, we investigated the distribution of CD34+ vessels inside of non-cancerous breast parenchyma, as well as in diabetic and non-diabetic breast cancer. As a result, we determined that diabetic status does not influence the amount of CD34+ blood vessels at the intra- and peritumoral sites.
Materials and methods
Patients. We have investigated immunohistochemically 58 cases of invasive ductal breast carcinomas of NST type (during 2021-2022, Institute of Oncology, Republic of Moldova). In 29 cases, the tumor was associated with DM type 2. The mean age was 63.2±6.5 years in the group of patients with diabetes mellitus type 2 and 64.5±7.9 years in the non-diabetic group. All patients underwent a Madden modified radical mastectomy with lymph nodes dissection, without prior chemo- and radiotherapy. Pre-operative fasting blood sugar level was measured by colorimetric method.
Ten samples (breast) of women which died accidentally served as control group. The mean age was 64.2±6.2.
Tissue processing and immunohistochemistry. The specimens were fixed in 10% phosphate buffered formalin for 24-48h and paraffin (Tissue-Tek Paraffin Wax TEK III) embedded as traditionally (Tissue-Tek VIP 6 AI, Sakura Finetek, USA). 3-5 μm sections were cut for histopathological assessment (Accu-Cut® SRM™ 200 Rotary Microtome, Sakura Finetek, USA), stained with Carazzi hematoxylin (Bio-Optica, Italy) and eosin Y (05-M10007, Bio-Optica, Italy) and mounted (Tissue-Tek Glas g2 Glass Coverslipper, Sakura Finetek, USA). The histological grading of breast carcinoma was performed according to the Nottingham system, which takes into consideration tubules and glandular/acini formation, nuclear pleomorphism and mitosis [10]. The Ft of Nottingham score was evaluated by evaluating tubules and glandular/acini formation: score 1 – majority (>75%) form tubules and glandular/acini, score 2 – moderate (10-75%), score 3 – low (<10%) or none tubules and glandular/acini formation.
The evaluation of mitotic activity was made according to WHO (2019) recommendation, as follows (x400): score 1 – nuclei are very similar in size to the nuclei of benign pre-existing epithelial cells (< 1.5 times the size), with minimal pleomorphism, nucleoli are either not visible or very inconspicuous; score 2 – nuclei are 1.5-2 times bigger in size, with mild to moderate pleomorphism, with visible, small and inconspicuous nucleoli; score 3 – nuclei larger 2 times than the size of benign epithelial cell nuclei, with vesicular chromatin, with high variation in size and shape, often with prominent nucleoli.
In the present study we used pathological TNM staging, which is based on tumor size, the number of affected lymph nodes and the presence of distant metastases [11].
The histopathological diagnosis was assessed by two pathologists and suitable for immunohistochemistry cases were carefully selected. Heat-induced epitope retrieval (microwave, 900W, 95°C, 20 min, followed by cooling at RT for 20 min) was made in citrate buffer (10mM Citric Acid, 0.05% Tween 20, pH 6.0). Incubation with primary monoclonal antibody (CD34 Ab1, RTU, 30 min, clone QBEND/10, NeoMarkers, Fremont, CA) was followed by Novolink Max Polymer Detection System (RE7280-K, 15 min, Leica Biosystems, DE). Specimens were processed automatically on Dako Autostainer Link 48 system. The Mayer’s hematoxylin, Lille’s modification (HMM500, ScyTek Laboratories) was used for counterstaining.
Microscopic evaluation. Microvessels amount was determined by hotspot approach [12]. Counts were made in ten fields (intra- and peritumoral or periductal/periacinar in case of control group, x200) containing the most abundant vascularity and the mean value (+/- SD) was determined. Any stained endothelial cells from adjacent vessels were counted as a single vessel, even in the absence of lumen.
Image acquisition and data processing. Slides were examined with Zeiss AxioImager 2.0 microscope with AxioCam Mrc5 installed camera by using ZEN core 3.5 imaging software.
Statistical analysis. Cases were grouped (MS Access 2007) into diabetic and non-diabetic groups by taking into account clinical and morphological data. The WINSTAT 2012.1 (R. Fitch Software, Bad Krozingen, Germany) software was used for descriptive statistics (M±SD, the median). The Spearman correlation (rs) was used to determine the relationship between different variables. The CD34 values from 2 groups were compared by t-independent test. For all tests a value of p£0.05 was considered statistically significant.
Ethics. This study has been approved by the Ethics Committee of the Nicolae Testemitanu University of Medicine and Pharmacy (nr.7, 12.11.2021).
Results
Morphologically normal breast tissue contained dense concentric network of CD34 positive vessels in the intra- and interlobular area, around glandular ducts and acini (Fig.1). The extralobular stroma harbored few CD34+ fibrocytes, which mainly surrounded thick-walled arteries. Slight CD34 staining was noted on small caliber blood vessels within the stroma. The mean of CD34 positive vessels in the periductal/periacinar region was estimated as 24.8±11.2 (ranging between 16 – 45, with 14 as median). No CD34 reactivity was observed inside of epithelial sheath.
In case of invasive ductal carcinoma, without associated diabetes, blood vessels with small, thin wall were haphazardly distributed within tumoral nests. The peritumoral areas contained blood vessels of medium caliber. Comparing the border between breast tumor and cancer-free zone, the abrupt loss of CD34+ blood vessels was observed. The intratumoral content of CD34+ vessels was evaluated as 10.5±8.3 (range 0 – 30, median equal to 9). At the peritumoral site, the number of CD34+ cells was 14±8.5 (range 2 – 45, the median as 14). These values can be considered similar, because no differences were determined in case of t-independent (t=-1.6, p=0.12), as well as t-dependent tests (t=-1.58, p=0.13).
In case of carcinoma associated with diabetes the mean of CD34+ vessels was 12.9±12.7 (0 – 59, median as 12) in the intratumoral site and 16±7.3 (0 – 27, 16 for median) in the peritumoral region. By comparing these areas we could not find statistically significant differences in case of t-independent (t=-1.17, p=0.25), as well as in case of t-dependent tests (t=-1.19, p=0.25).
Single statistically significant correlation was determined at the intratumoral site (table 1). In case of tumors with normal sugar level in the blood, intratumoral expression of CD34 correlated statistically significant with lymphovascular invasion (rs=0.34, p=0.03) and pT stage (rs=0.54, p=0.001).
Table 1. Spearman correlation between CD34 expression, patients’ age, glucose level and tumor’s features | ||||||||
| CD34it | CD34pt | ||||||
Cancer | Cancer + Diabetes | Cancer | Cancer + Diabetes | |||||
rs | p | rs | p | rs | p | rs | p | |
Patients age | -0.08 | 0.34 | 0.07 | 0.36 | -0.30 | 0.06 | 0.17 | 0.19 |
Sugar level | 0.06 | 0.38 | 0.0 | 0.49 | 0.04 | 0.42 | 0.01 | 0.48 |
Nottingham grade | -0.04 | 0.42 | -0.34 | 0.04 | 0.19 | 0.17 | -0.17 | 0.19 |
Ft | -0.01 | 0.48 | -0.34 | 0.04 | 0.23 | 0.11 | 0.16 | 0.20 |
Nuclear atypia | -0.09 | 0.32 | 0.0 | 0.49 | 0.04 | 0.42 | -0.23 | 0.11 |
Mitotic activity | 0.05 | 0.39 | -0.22 | 0.13 | 0.12 | 0.27 | -0.19 | 0.16 |
Lymphovascular invasion | 0.34 | 0.03 | -0.04 | 0.41 | -0.06 | 0.38 | -0.11 | 0.29 |
Perineural invasion | 0.0 | 0.50 | 0.23 | 0.12 | 0.21 | 0.14 | 0.23 | 0.11 |
pT | 0.54 | 0.001 | -0.20 | 0.15 | 0.11 | 0.28 | 0.17 | 0.18 |
pN | 0.30 | 0.06 | 0.18 | 0.18 | -0.09 | 0.32 | -0.07 | 0.36 |
CD34it |
|
|
|
| 0.02 | 0.46 | -0.16 | 0.20 |
Note: CD34it – CD34 positive vessels at the intratumoral site; CD34pt – at the peritumoral site; rs – Spearman correlation; p – statistical significance; Ft – Nottingham’s score Ft; pT – pathologic stage of tumor; pN – pathologic stage of lymph node metastases. With Bold are selected the statistically significant correlations. | ||||||||
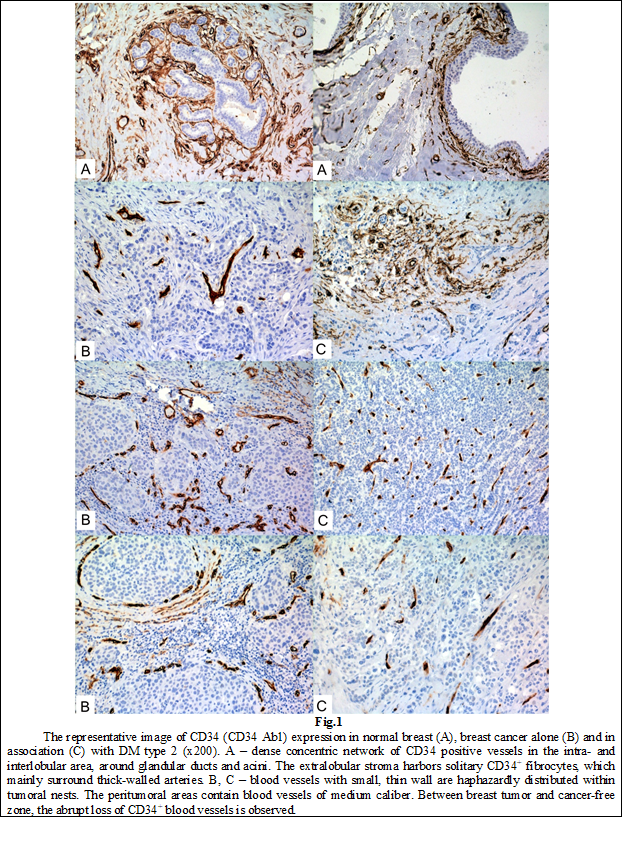
In case of tumors associated with DM, CD34+ vessels amount of intratumoral site negatively correlated with tumors grade and Ft (rs=-0.34, p=0.04). No statistically significant correlations were determined in case of peritumoral expression of CD34 in both groups.
Comparing both groups, only sugar level (p=0.0001) and Ft (p=0.05) values were statistically different (table 2).
Table 2. The differences between breast cancers, alone or in association with DM type 2 | |
| Student independent t-Test (p) |
Patients age | 0.51 |
Sugar level | 0.0001 |
Nottingham grade | 0.29 |
Nuclear atypia | 0.79 |
Mitotic activity | 0.48 |
Lymphovascular invasion | 0.41 |
Perineural invasion | 0.34 |
pT | 0.79 |
pN | 0.60 |
Ft | 0.05 |
Nuclear atypia | 0.81 |
CD34pt | 0.34 |
CD34it | 0.41 |
Note: p – statistical significance; pT – pathologic stage of tumor; pN – pathologic stage of lymph node metastases; Ft – Nottingham’s score Ft; CD34it – CD34 positive vessels at the intratumoral site; CD34pt – at the peritumoral site. With Bold are selected the statistically significant differences. | |
Discussions
There are many experimental evidences to prove that tumor growth and future prognosis are dependent on angiogenesis [13]. The expansion of the tumor cells population requires the generation of new vessels, which in turn increase the opportunity of tumor to develop metastases. Thus, the intensity of angiogenesis can predict the future tumor’s behavior and stimulate the expansion throughout body. Different studies have used diverse markers to highlight blood vessels in breast tumors in order to describe and measure angiogenesis.
CD34, also known as human hematopoietic progenitor cell antigen, is a constitutive stroma component of most tissues, including female breast [5]. It is a single-chain transmembrane glycoprotein with a molecular weight of 105-120 kDa, located on the long arm of chromosome, predominantly expressed on endothelial and hematopoietic progenitor cells, and closely associated with the process of angiogenesis [6, 12]. Nowadays, there are many studies, which have been conducted to investigate the distribution of CD34+ stromal cells and vessels in neoplasms of various organs including salivary gland, stomach, colorectal tissue, breast, pancreas, uterine cervix and mammary gland [4, 13-15].
A total of 382 million people had diabetes in 2013 and this number is expected to rise to 592 million by 2035 [15]. About 16% of breast cancer patients suffer from diabetes and the last can be associated with 10-20% excess relative risk of breast cancer [16]. This opinion is in line with Giovannucci et al. (2010) data, which supports that diabetes can increase the risk of tumor development [17]. A well-designed meta-analysis provided by Bruijn et al. (2013) shows that women with diabetes had a 23% greater risk of subsequent breast cancer than those without diabetes [18].
CD34 has a diverse distribution in the tumoral stroma and it is evident that we have to consider carcinogenesis and tumor progression as a multicellular interaction within newly formed tissue, so called cancer tissue. Although the role of CD34, as a marker of angiogenesis in tumors is still unclear, there are many evidences that increase of CD34+ vessels associates with a high risk of metastasis development [19]. Fathy et al. (2018) demonstrated that high CD34 expression is statistically significant associated with tumor size and stage, as well as lymph nodes metastasis development in cervical cancer [20]. Authors consider that high expression of CD34 signifies the presence of an anomalous vessel pattern and disturbing tumor vasculature is one of the primary strategies in cancer chemotherapy. Moreover, this marker has an important diagnostic and prognostic value – the decrease of CD34 expression after therapy correlates with its effectiveness [21].
The assumption before making this study was that DM affects blood vessels and this can alter the tumor’s grow. DM is characterized by poor circulation and impaired angiogenesis, which appear to contribute to lesions and poor wound healing. Lega et al. (2018) consider that patients with associated DM have a poorer prognosis because of estrogenic effects of obesity or metabolic factors like growth-promoting influence of hyperinsulinemia and insulin resistance [22]. In case of present study, we couldn’t find any numerical differences between vessels density, at intra- and peritumoral sites, caused by DM. It looks like, the tumor progression occurs in the similar way, in spite of all lesions caused by DM.
The effect of DM on normal tissues does not resume only on sugar level and atherosclerosis. D’Alessandra et al. (2021) determined that diabetes induces a transcriptional signature in bone marrow-derived CD34+ hematopoietic stem cells [23]. Specifically, these cells displayed reduced expression of genes coding for proteins regulating antibacterial and antivirus host defense as well as macrophage differentiation and lymphocyte emigration, proliferation, and differentiation. Moreover, a consistent number of inflammatory genes coding for chemokines and cytokines were up-regulated.
The existing reports regarding the amount of vessels are still controversial, which led us to examine the distribution of CD34+ vessels in the intra- and peritumoral stroma in diabetic and non-diabetic patients. Jarajapu et al. (2014) demonstrated that DM patients have microvascular complications, which exhibit severely limited capacity to generate ex-vivo expanded endothelial progenitor populations [24]. Moreover, vasoreparative dysfunction observed in diabetic CD34+ cells is due to impaired autocrine/paracrine function and reduced sensitivity to hypoxia. In Durrani et al. (2021) opinion, association of DM type 2 worsen breast cancer prognosis and may decrease body defense capacity by changing tumoral microenvironment under disturbed hormonal activity by altering endogenous sex-hormone regulation and activation of the IGF and insulin-signaling pathways [25]. Rask-Madsen et al. (2013) consider that DM background alters blood vessels permeability at the level of microcirculation, statement, which let us initially to launch hypothesis that intra- and peritumoral environment of breast cancer, alone or in DM association, can be different [26].
This study has some limitations. First, it does not reflect the general population, because only women of 50 years and older have been taken. Secondly, we did not measure the sugar level in case of control group. Thirdly, we have no data about diabetes duration, a factor that in Zoungas et al. (2014) opinion is directly associated with macro- and microvascular changes [27]. Another limitation is the absence of a cut-off for CD34 expression, which splits the cases into the high/low expression [6]. Moreover, we did not take into consideration the status of lymph nodes.
Conclusions
The expression of CD34 in breast cancer stroma is not homogenous, irrespective of association with diabetes mellitus type 2. Question if breast carcinoma and diabetes mellitus are concurrent or associated disorders remains open. Probably, the effect of carcinoma prevails in influencing the structure of the tumor microenvironment. We expect a further confirmation in larger study groups.
Competing interests.
None declared.
Authors’ contribution
VF, VD and LS elaborated the hypothesis, design of the study and had a significant intellectual contribution in data interpretation and discussion of the results. DB, EP and EC gathered primary material, processed and described, counted blood vessels performed the statistical analysis. EF, DB and VF wrote the draft of the study. All authors have read and approved the final version of the manuscript.
Authors’ ORCID IDs
Ecaterina Foca – https://orcid.org/0000-0001-7629-4875
Dumitru Brinza – https://orcid.org/0000-0002-3133-1502
Elena Portnoi – https://orcid.org/0000-0002-0168-7102
Ecaterina Carpenco – https://orcid.org/0000-0003-1464-3149
Valeriu David – https://orcid.org/0000-0001-9799-7369
Lilian Saptefrati – https://orcid.org/0000-0003-2779-718X
Veaceslav Fulga – https://orcid.org/0000-0002-7589-7188
References.
Mafu TS, September AV, Shamley D. The potential role of angiogenesis in the development of shoulder pain, shoulder dysfunction, and lymphedema after breast cancer treatment. Cancer Manag Res. 2018;10:81-90. doi: 10.2147/CMAR.S151714.
Mao Y, Keller ET, Garfield DH, Shen K, Wang J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013 Jun;32(1-2):303-15. doi: 10.1007/s10555-012-9415-3.
Angeli F, Koumakis G, Chen MC, Kumar S, Delinassios JG. Role of stromal fibroblasts in cancer: promoting or impeding? Tumour Biol. 2009;30(3):109-20. doi: 10.1159/000218708.
Martinez LM, Labovsky V, de Luján Calcagno M, Davies KM, Rivello HG, Bianchi MS, et al. CD105 expression on CD34-negative spindle-shaped stromal cells of primary tumor is an unfavorable prognostic marker in early breast cancer patients. PLoS One. 2015 Mar;10(3):e0121421. doi: 10.1371/journal.pone.0121421.
Yamazaki K, Eyden BP. Ultrastructural and immunohistochemical observations on intralobular fibroblasts of human breast, with observations on the CD34 antigen. J Submicrosc Cytol Pathol. 1995 Jul;27(3):309-23.
Chen Z, Xu S, Xu W, Huang J, Zhang G, Lei L, et al. Expression of cluster of differentiation 34 and vascular endothelial growth factor in breast cancer, and their prognostic significance. Oncol Lett. 2015 Aug;10(2):723-9. doi: 10.3892/ol.2015.3348.
Sundov Z, Tomic S, Alfirevic S, Sundov A, Capkun V, Nincevic Z, et al. Prognostic value of MVD, LVD and vascular invasion in lymph node-negative colon cancer. Hepatogastroenterology. 2013 May;60(123):432-8. doi: 10.5754/hge12826.
Anderson GF, Chu E. Expanding priorities – confronting chronic disease in countries with low income. N Engl J Med. 2007 Jan;356(3):209-11. doi: 10.1056/NEJMp068182.
Kaplan MA, Pekkolay Z, Kucukoner M, Inal A, Urakci Z, Ertugrul H, et al. Type 2 diabetes mellitus and prognosis in early stage breast cancer women. Med Oncol. 2012 Sep;29(3):1576-80. doi: 10.1007/s12032-011-0109-4.
International Agency for Research on Cancer. Publication of the WHO Classification of Tumours, 5th Edition, Volume 2: Breast Tumours [Internet]. Lyon: IARC; 2023- [cited 2022 Dec 14]. Available from: https://www.iarc.who.int/news-events/who-classification-of-tumours-5th-…
Brierley J, Gospodarowicz M, Wittekind C, editors. TNM classification of malignant tumours. 8th ed. [Internet]. Hoboken: John Wiley & Sons; 2017 [cited 2022 Dec 14]. Available from: https://www.uicc.org/resources/tnm-classification-malignant-tumours-8th…
Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991 Jan;324(1):1-8. doi: 10.1056/NEJM199101033240101.
Zuazo-Gaztelu I, Casanovas O. Unraveling the role of angiogenesis in cancer ecosystems. Front Oncol. 2018;8:248. doi: 10.3389/fonc.2018.00248.
Westhoff CC, Jank P, Jacke CO, Albert U-S, Ebrahimsade S, Barth PJ, et al. Prognostic relevance of the loss of stromal CD34 positive fibroblasts in invasive lobular carcinoma of the breast. Virchows Arch. 2020 Nov;477(5):717-24. doi: 10.1007/s00428-020-02835-3.
Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014 Feb;103(2):137-49. doi: 10.1016/j.diabres.2013.11.002.
Wolf I, Sadetzki S, Catane R, Karasik A, Kaufman B. Diabetes mellitus and breast cancer. Lancet Oncol. 2005 Feb;6(2):103-11. doi: 10.1016/S1470-2045(05)01736-5.
Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010 Jul;33(7):1674-85. doi: 10.2337/dc10-0666.
De Bruijn KMJ, Arends LR, Hansen BE, Leeflang S, Ruiter R, van Eijck CHJ. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br J Surg. 2013 Oct;100(11):1421-9. doi: 10.1002/bjs.9229.
Kademani D, Lewis JT, Lamb DH, Rallis DJ, Harrington JR. Angiogenesis and CD34 expression as a predictor of recurrence in oral squamous cell carcinoma. J Oral Maxillofac Surg. 2009 Sep;67(9):1800-5. doi: 10.1016/j.joms.2008.06.081.
Fathy A, Abdelrahman AE. EZH2, Endothelin-1, and CD34 as biomarkers of aggressive cervical squamous cell carcinoma: an immunohistochemical study. Turk Patoloji Derg. 2018;34(2):150-7. doi: 10.5146/tjpath.2018.01425.
Lin Y, Li Z, Liu M, Ye H, He J, Chen J. CD34 and Bcl-2 as predictors for the efficacy of neoadjuvant chemotherapy in cervical cancer. Arch Gynecol Obstet. 2021 Aug;304(2):495-501. doi: 10.1007/s00404-020-05921-8.
Lega IC, Austin PC, Fischer HD, Fung K, Krzyzanowska MK, Amir E, et al. The impact of diabetes on breast cancer treatments and outcomes: a population-based study. Diabetes Care. 2018 Jan;41(4):755-61. doi: 10.2337/dc17-2012.
D’Alessandra Y, Chiesa M, Vigorelli V, Ricci V, Rurali E, Raucci A, et al. Diabetes induces a transcriptional signature in bone marrow–derived CD34+ hematopoietic stem cells predictive of their progeny dysfunction. Int J Mol Sci. 2021 Jan;22(3):1423. doi: 10.3390/ijms22031423.
Jarajapu YPR, Hazra S, Segal M, LiCalzi S, Jhadao C, Qian K, et al. Vasoreparative dysfunction of CD34+ cells in diabetic individuals involves hypoxic desensitization and impaired autocrine/paracrine mechanisms. PLoS One. 2014 Apr;9(4):e93965. doi: 10.1371/journal.pone.0093965.
Durrani IA, Bhatti A, John P. The prognostic outcome of ‘type 2 diabetes mellitus and breast cancer’ association pivots on hypoxia-hyperglycemia axis. Cancer Cell Int. 2021 Jul;21(1):351. doi: 10.1186/s12935-021-02040-5.
Rask-Madsen C, King GL. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab. 2013 Jan;17(1):20-33. doi: 10.1016/j.cmet.2012.11.012.
Zoungas S, Woodward M, Li Q, Cooper ME, Hamet P, Harrap S, et al. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia. 2014 Dec;57(12):2465-74. doi: 10.1007/s00125-014-3369-7.