Introduction
Global consumption of plant protein has increased by 15% since the early 1960s, and positive health benefits of plant foods are linked to dietary fiber, vitamins, minerals, and phytochemicals. The food industry is developing plant-derived proteins as an alternative to meat and animal-derived proteins to meet consumer demand, which is largely based on cultural, dietary and religious choices [1].
The pharmaceutical industry uses vegetal proteins to treat various diseases such as rheumatoid arthritis, coronary thrombosis, multiple sclerosis and chronic lymphocytic leukaemia. Thus, pharmacological actions, such as: the potential to reduce the risk of developing metabolic syndrome or to manage diabetes and prevent cancer, are studied and discussed, as well as the different grading systems currently used to determine the quality of proteins from plant sources [1, 2]. Plant-derived proteins have increased bioavailability and may be more concentrated than animal protein sources [2, 3].
Despite its benefits, proteins, especially plant proteins, are also known as major sources of allergens that trigger an allergenic response via immunoglobulin E (Ig E)-mediated allergies [4, 5]. These allergens may diffuse into the body from the upper respiratory tract or enter the body through intake of vast range of plant food or may cause external skin irritations. Allergens mainly present in pollen, spores, and other plant associated products are responsible for symptoms such as rhinoconjunctivitis, asthma, edema, urticarial, and anaphylaxis [6].
The prevalence of allergic diseases has increased, especially in the last 20 years in most countries, with manifestations through gastrointestinal pathologies, atopic eczema, and asthma [6], pollen being one of the main allergens [7]. Allergic reactions to legumes by inhalation have rarely been described [8], however, there are studies describing the occurrence of allergic reactions manifested by rhinoconjunctivitis and bronchial asthma [9]. The most widespread groups of plant allergens that are reported belong to the seed storage proteins, structural proteins, and pathogenesis related proteins, induced as a defense response system under stressful conditions such as infections, insects, injuries, exposure to harsh chemicals and atmospheric conditions [10, 11]. In this context, we aimed to determine the molecular weight and the total protein concentration in some dried extracts obtained from plants: Agrimony, Chicory, Lady's bedstraw, and European goldenrod.
Agrimony (Agrimonia eupatoria L., Rosaceae). Among the major phytochemical constituents of this species are polyphenols: phenolic acids: p-coumaric acid, various caffeoyl-quinic acids [12]; flavonoids: luteolin, apigenin, quercetin, and kaempferol derivatives, including C-glycosides such as vitexin and iso-vitexin, which are derived of apigenin; procyanidins: catechin, procyanidins and ellagitannins-mainly agrimoniin [13]. Agrimony has been used as a remedy for a wide range of diseases and symptoms: antioxidant, inflammatory diseases [14], ailments of the gastrointestinal tract, including diarrhea and stomatitis, in liver disorders [15]. It is claimed to have antibacterial activity [16], particularly on Gram-positive species and, rather inappropriately, on probiotic species. Neuroprotective, hepatoprotective [17], diuretic [18], anti-inflammatory, through cytokine modulation [19], and anti-nociceptive, wound healing and cytotoxic [20] effects were also reported for various extracts, fractions or individual compounds isolated from A. eupatoria. Plants of the g. Agrimony, mainly used as pharmaceutical raw material, perfectly fit into the current trends in technology that are searching for organic raw materials with high contents of bioactive compounds, such as dietary polyphenols and fiber [21, 22].
Chicory (Cichorium intybus L.; Asteraceae) is also a medicinal plant with a long tradition of use across various geographic regions. The aerial parts of Chicory contain cichoriin, arginine, choline, chicoric acid, bitter principles, and microelements: Fe, P, Ca. The entire plant contains latex, whose major constituent is inulin- type fructans. Besides inulin, there are also sugars, tannins, essential and fixed oils, pectin, and resins. The plant also serves as a source of vitamins: A, C, E, K, PP, flavonoids make up about 3%. The roots are rich in bitter triterpenic substances, fructose, tannins, and essential oils [23, 24]. Its aerial parts have been used as salads, its roots have been employed as a coffee substitute, and all its parts have been attributed a variety of potential health benefits: anti-inflammatory, hypolipidemic, gastroprotective, analgesic, antidiabetic, reproductive enhancing, wound healing, anticancer, antimicrobial, and anthelmintic and others [24, 25]. Chicory is found in the food industry: as a salad, for teas, food supplements, coffee supplements or as a source for inulin production [26]. Some compounds present in chicory, such as polyphenols, hydroxycinnamic acids, inulin, protein, can be considered as potential carriers of food functionality, the main biological activities of the species being associated with the presence of bioactive compounds [27].
Lady's bedstraw (Galium verum L., Rubiaceae), a species that blooms on the summer solstice and has a special role in the traditions and spirituality of our people, but in the Republic of Moldova they are not studied until the present [28]. Based on investigations and phytochemical studies carried out, in the aerial parts of G. verum have been identified phenolic compounds, flavonoids (rutin, quercetin, isoquercitrin, apigenin, myricetin, luteolin, kaempferol); hydroxycinnamic acids (gentisic, caffeic, chlorogenic, p-coumaric, ferulic, sinapic, caftaric, rubiforic [29, 30]. The entire plant contains iridoid glycosides: asperulozide, asperulozidic acid, diacetyl-asperulozide, 3,4-dihydro-3-methoxi-asperuloside, monotropeine, acetyl-dafiloside and scandoside [31]. Anthracene derivatives, essential oils, tannins, saponosides and coumarins were also identified in smaller quantities [30]. The literature indicates that total polyphenols depend on both the nature and concentration of the solvent and the extraction technique applied, with large differences ranging from (2.44-5.16) mg/g in the dried plant product of G. verum, [32], up to 75.3 mg/g in the methanolic extract [33]. Regarding the application of different extraction techniques: ultrasonic, maceration, reflux, it was demonstrated that the highest amount of phenolic compounds was obtained by applying the ultrasonic extraction method [28]. Previous pharmacological studies have shown that species of g. Galium possess antioxidant, diuretic, spasmolytic, cytotoxic, antimicrobial, endocrine and protective effects [30, 34].
European goldenrod (Solidago virgaurea L., Asteraceae) is widely used in traditional medicine and among the most researched species from g. Solidago [35]. The aerial parts of European goldenrod have long been used for urinary tract conditions as a diuretic and a disinfectant remedy and as an anti-inflammatory agent [36]. According to the latest research in the field and besides the fact of the positive effect on the urinary system, herb and extracts also manifest antioxidant, analgesic, antibacterial, antifungal, antispasmodic, immunostimulant, antiadipogenic and antidiabetic activities [35, 37-39]. The chemical profile of the plant is a various one, being mostly represented by flavonoids (mainly derived from quercetin and kaempferol), terpenes (mostly from the essential oils), and saponosides (mainly virgaureasaponins and solidagosaponins [35, 40-44]. Due to overexploitation of the plant in its natural habitats, this taxon has become a rare species in Europe. Currently, European goldenrod is cultivated in some European countries thanks to scientific agronomic research. Therefore, the plant micropropagation method and other in vitro growth systems allow the multiplication of plant biomass for phytochemical and biological research [36].
Materials and methods
The vegetal products: Agrimoniae herba, Cichorii herba, Galii herba, Solidaginis virgaureae herba were harvested from the collection of the SPCFMP, according to the nature of the herbal products, throughout the flowering period. The vegetal products have been processed in agreement to recommendations for the purposes of chemical studies. The powdered drying herbal products was passed through a sieve with the dimensions of 0,5 mm. Extracts were obtained by repeatedly extracting the plant products sprayed with a mixture of ethanol: water (60%, w/w) for half an hour at each extraction stage, until the plant products were exhausted, with extraction applied by magnetic stirring. The extractive solutions thus obtained were concentrated at 40 °C, using a rotary evaporator- Laborota 4011 [22].
Protein extraction was carried out on the dry extract of four vegetal products: Agrimoniae herba, Cichorii herba, Galii veri herba and Solidaginis virgaureae herba. The proteins were extracted using four extraction buffers: Tris-Glycine (pH 8.3), PBS (Phosphate-buffered saline with pH 7.4), Citrate buffer (pH 4.5) and Carbonate-bicarbonate buffer (pH 9.6). Each extract was weighed twice at 150 mg and added 750 ml of extraction solution. Samples were shaken and placed in a thermomixer: 600 rpm at 45 ºC, to exclude protein denaturation, for 3 hours. Every 30 minutes, the samples were vortexed. The supernatant was collected in a 2 ml Eppendorf tube and refrigerated overnight.
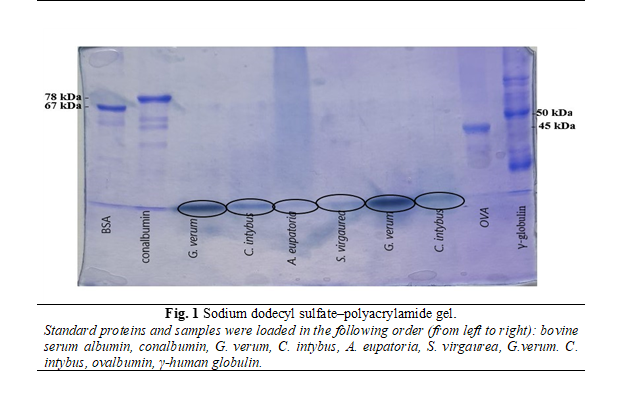
Qualitative determination of proteins was performed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and total content of proteins were determined using Bradford assay [45].
SDS-PAGE allows separating proteins according to their molecular weight. At the beginning were prepared 2 sandwiches (a sandwich is made of an alumina ceramic plate, two spacers and one glass plate) and put them into gel caster. After, according to the pipetting scheme was prepared separation gel solution and filled it into the sandwiches with a Pasteur pipette (~ 6 cm high). Each of sandwiches were overlaid with 100 μl of water saturated 1-butanol and left for 30 minutes to polymerize. The stacking gel solution, prepared according to the pipetting scheme, was pipetted into both sandwiches and were introduced the combs. After another 30 min of polymerization, the sandwiches were separated with a knife and marked the samples loading wells on the glass plate. The samples were prepared by taking 30 μl of standard or the fractions to be analyzed and mixed with the same amount of denaturation mix and heated it for 5 min at no more than 60°C [46].
The next step was electrophoresis preparation. Each sandwich was attached to the electrophoresis unit with two clamps, the cooling tubes were connected, and the water tap was turned on. The lower chamber was filled with buffer solution (sandwiches should be submerged about 1 cm), the comb was removed and the upper chamber was filled with buffer solution (behind the sandwiches). Loaded 5 μl of the standard proteins BSA (67 kDa), human γ-globulin (50 kDa), conalbumin (78 kDa), ovalbumin (45 kDa) and the samples, placed the safety lid onto the electrophoresis unit and plugged to a power supply. At the beginning, the current was 40 mA and the run took about 1 h. After the time elapsed, the sandwiches were lifted and the stacking gel was separated from the glass plate and placed in a tray filled with staining solution. Staining was carried out at 40°C for 30 min. The next step was destaining the gels using two solutions [47].
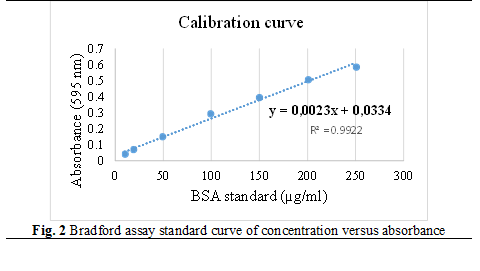
The Bradford assay is a spectrophotometric method, which is used to measure the concentrations of proteins according to the absorbance of Coomassie Brilliant Blue G-250 dye at 595 nm. The following dilutions were prepared from the BSA (bovine serum albumin) standard solution at a concentration of 1mg/ml to make the calibration curve: 500, 250, 200, 200, 150, 100, 50, 20, 10 μg/ml. Samples were diluted 1:100, 1 ml of Bradford working solution was added and incubated for 5 minutes. Thereafter, absorbance was measured at 595 nm [45, 48] by performing 5 technological repeats.
Results
Extracts obtained from the aerial parts of A. eupatoria, C. intybus, G. verum and S. virgaurea, in a mixture of ethanol: water (60%, w/w) until the vegetal products were exhausted and concentrated at 40°C using a rotary evaporator, were analyzed proteins according to the Protein Protocols Handbook [49].
Analyzing the obtained gel (figure 1), we confirm the presence of proteins in the extracts, but in all samples the proteins had a lower weight than used standards. The thickest bands belong to the extracts of G. verum and C. intybus and the thinnest to A. eupatoria and S. virgaurea.
Protein concentration was calculated through Bradford assay, from the calibration curve of concentration versus absorbance in µg/ml (figure 2).
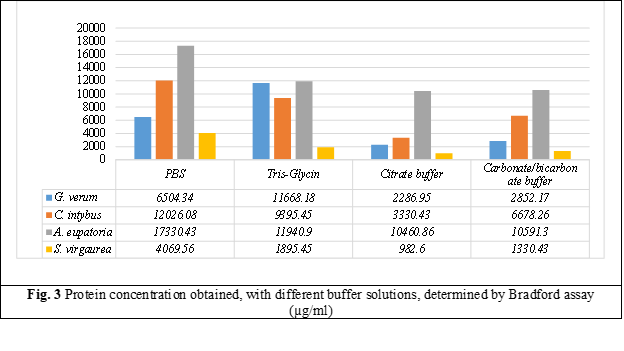
Protein extraction at different pH of buffer solutions showed that better protein extraction is achieved with neutral pH buffer, and with increasing buffer’s pH in basic, the concentration dcreases. Weaker protein extraction is observed with the acidic citrate buffer at pH 4.5.
As a result, a better protein extraction was obtained with Phosphate-buffered saline (PBS) extraction buffer (pH 7.4) which showed higher protein values in all extracts examined; followed by Tris-Glycin (pH 8.3), Carbonate-bicarbonate (pH 9.6) and Citrate (pH 4.5) extraction buffers. Analyzing the obtained results, we can conclude that a higher concentration of protein is found in the dry extract of A. eupatoria, followed by C intybus, G. verum and a lower concentration of protein we have in the dry extract of S. virgaurea.
Discussion
It is well known that the field of modern medicines has recently increasingly focused its interest on herbal medicines with minor side effects. The exploitation of natural resources used in particular in folk tradition is of particular interest because of their biological potential, and the pharmacotherapeutic value of plants is linked to their phytochemical components and secondary metabolites.
The Bradford spectrophotometric test (absorbance 595 nm) used to measure protein concentrations as a function of absorbance of Coomassie Brilliant Blue G-25 dye show that, the richest in protein is the dry extract of Agrimony, with a protein maximum of 17330.43 µg/ml extracted with PBS buffer, followed by 11940.9 µg/ml with Tris-Glycin buffer, 10591.3 extracted by Carbonate-bicarbonate buffer and a concentration of 10460.86 with Citrate buffer.
The second plant, by protein concentration, is Chicory. The best protein extraction was obtained with PBS buffer 12 026.08 µg/ml, followed by Tris-Glycin buffer with 9395.45 µg/ml, 6678.26 µg/ml with Carbonate-bicarbonate buffer and last with Citrate buffer with a protein concentration of 3330.43 µg/ml.
The third plant, by protein concentration, is Lady's bedstraw. The dry extract of this plant showed a higher protein extraction with Tris-Glycin buffer, with a protein concentration of 11668.18 µg/ml, followed by 6504.34 µg/ml with PBS buffer, 2852.17 µg/ml with Carbonate-bicarbonate buffer and a lower protein value was received with Citrate buffer 2286.95 µg/ml.
A lower protein concentration had European goldenrod. All buffer solutions used, showed a low protein concentration. Extraction with PBS solution showed a higher concentration of protein 4069.56 µg/ml, followed by Tris-Glycin with 1895.45 µg/ml of proteins, 1330.43 µg/ml with Carbonate-bicarbonate buffer solution and a lower concentration was determining with Citrate buffer 982.6 µg/ml.
The low protein concentrations in dried plant extracts are due to the ethyl alcohol used as an extraction agent. Studies show that proteins have much lower solubility in polar solvents such as ethanol [50].
Conclusions
The development of the pharmaceutical and beneficial food industry branches for health and personalised treatment requires careful evaluation of phytocompounds in products to ensure the positive effect on human health and to minimise consequences and risks.
The results obtained show a low protein concentration, which suggests a potential lack of allergic reactions and intolerance to the given plant extracts for A. eupatoria, C. intybus, G. verum and S. virgaurea.
List of abbreviations used
BSA – bovine serum albumin; OVA – ovalbumin; PBS– Phosphate-buffered saline; SPCFMP – Scientific Practical Center in the Field of Medicinal Plants; SDS-PAGE – sodium dodecyl sulfate–polyacrylamide gel electrophoresis; SUMPh – State University of Medicine and Pharmacy
Competenig interests
None declared
Authors’ contributions
AO collected the data, performed the experimental part; MCM coordinated experimental activity and approved the manuscript; MCT drafted and revised the manuscript; TC interpreted the data and drafted the manuscript; NC revised the manuscript critically; CF collected the data and drafted the manuscript; CC analysis of data; AB interpreted the data; LU interpreted the data and drafted the manuscript. All authors revised and approved the final version of the manuscript.
Acknowledgments
This study was supported by the Nicolae Testemitanu State University of Medicine and Pharmacy of the Republic of Moldova through the scientific research under the Moldovan State Program (2020–2023): „Biological and phytochemical study of medicinal plants with antioxidant, antimicrobial and hepatoprotective action” (no. 20.80009.8007.24) and „Complex research for the development of new local anti-infective pharmaceuticals for optimizing the pharmacotherapy of dental, oropharyngeal and auricular diseases” (no. 20.80009.8007.14). The experimental part was carried out in the Department of Analytical Chemistry, University of Vienna, Austria (CEEPUS mobility M-RO-0010-2122-153838, OA).
Authors’ ORCID IDs
Angelica Ohindovschi – https://orcid.org/0000-0001-5132-0782
Margit Cichna-Markl – https://orcid.org/0000-0001-8699-674X
Maria Cojocaru-Toma – https://orcid.org/0000-0002-8255-9881
Tatiana Calalb – https://orcid.org/0000-0002-8303-3670
Nicolae Ciobanu – https://orcid.org/0000-0002-2774-6668
Cornelia Fursenco – https://orcid.org/0000-0003-0692-6819
Cristina Ciobanu – https://orcid.org/0000-0001-6550-6932
Anna Benea – https://orcid.org/0000-0001-9670-5045
Livia Uncu – https://orcid.org/0000-0003-3453-2243
References
Ahnen RT, Jonnalagadda SS, Slavin JL. Role of plant protein in nutrition, wellness and health. Nutr Rev. 2019;77(11):735-747. doi: 10.1093/nutrit/nuz028.
Hayes M. Seaweeds: a nutraceutical and health food. In: Tiwari B, editor. Seaweed Sustainability. Cambridge: Academic Press; 2015. p. 365-387.
Wong KL, Wong RN, Zhang L, et al. Bioactive proteins and peptides isolated from Chinese medicines with pharmaceutical potential. Chin Med. 2014;9:19. doi: 10.1186/1749-8546-9-19.
Pudasainee P, Anjum F. Protein Intolerance. In: StatPearls [Internet]. Treasure Island: StatPearls Publishing; 2022 [cited 2023 Dec 23]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK562306/
Bokszczanin K, Przybyła A. Molecular aspects of allergy to plant products. Part I. Class I and II allergens and crossreactivity of IgE antibodies. Pol Merkur Lekarski. 2012;32(188):129-134.
Chandra R. Food hypersensitivity and allergic diseases. Eur J Clin Nutr. 2002;56(3):S54-S56.doi: 10.1038/sj.ejcn.1601487.
Marsh D, Lichtenstein L, Norman P. Induction of IgE-mediated immediate hypersensitivity to group I rye grass pollen allergen and allergoids in non-allergic man. Immunology. 1972;22(6):1013-28.
Martin J, Compaired J, de la Hoz B, et al. Bronchial asthma induced by chick pea and lentil. Allergy. 1992;47(2 Pt 2):185-7. doi: 10.1111/j.1398-9995.1992.tb00962.x.
Valdivieso R, Subiza J, Varela-Losada S, et al. Bronchial asthma, rhinoconjunctivitis, and contact dermatitis caused by onion. J Allergy Clin Immunol.1994;94(5):928-30. doi: 10.1016/0091-6749(94)90161-9.
Sinha M Singh RP, Kushwaha GS, et al. Current overview of allergens of plant pathogenesis related protein families. Sci World J. 2014;543195. doi: 10.1155/2014/543195.
Duffort O, Polo F, Lombardero M, et al. Immunoassay to quantify the major peach allergen Pru p 3 in foodstuffs. Differential allergen release and stability under physiological conditions. J Agric Food Chem. 2003;50(26):7738-41. doi: 10.1021/jf0258398.
Correia H, González-Paramás A, Amaral M, Santos-Buelga C, Batista M. Polyphenolic profile characterization of Agrimonia eupatoria L. by HPLC with different detection devices. Biomed Chromatogr. 2006;20(1):88-94. doi: 10.1002/bmc.533.
Granica S, Kluge H, Horn G, Matkowski A, Kiss A. The Phytochemical investigation of Agrimonia eupatoria L. and Agrimonia procera Wallr. as valid sources of Agrimoniae herba - the pharmacopoeial plant material. J Pharm Biomed Anal. 2015;114:272-9. doi: 10.1016/j. jpba.2015.05.027.
Muruzovic M, Mladenović K, Stefanović O, Vasić S, Čomić L. Extracts of Agrimonia eupatoria L. as sources of biologically active compounds and evaluation of their antioxidant, antimicrobial, and antibiofilm activities. J Food Drug Anal. 2016;24:539-47. doi:10.1016/j. jfda.2016.02.007.
Ginovyan M, Trchounian A. Novel approach to combat antibiotic resistance: evaluation of some Armenian herb crude extracts for their antibiotic modulatory and antiviral properties. J Appl Microbiol. 2019;127:472-80. doi: 10.1111/jam.14335.
Nicu A, Pîrvu L, Vamanu A. Antibacterial activity of ethanolic extracts from Agrimonia eupatoria L. and Epilobium hirsutum L. herba. Sci Bull. Series F. Biotechnol. 2017;21:127-32.
Lee K, Hwang L, Jeong E, Kim S, Kim Y, Sung S. Effect of neuroprotective flavonoids of Agrimonia eupatoria on glutamate-induced oxidative injury to HT22 hippocampal cells. Biosci Biotechnol Biochem. 2010;74(8):1704-6.doi: 10.1271/bbb.100200.
Giachetti D, Taddei E, Taddei I. Diuretic and uricosuric activity of Agrimonia eupatoria L. Boll Soc Ital Biol Sper. 1986;62:705-711.
Ivanova D, Vankova D, Nashar M. Agrimonia eupatoria tea consumption in relation to markers of inflammation, oxidative status and lipid metabolism in healthy subjects. Arch Physiol Biochem. 2013;119(1):32-7. doi: 10.3109/13813455.2012.729844.
Adhiah A, Al-Bederi O, Al-Sammarrae K. Cytotoxic effects of Agrimonia eupatoria L. against cancer cell lines in vitro. J Assoc Arab Univ Basic Appl Sci. 2013;14(1):87-92. https://doi.org/10.1016/j.jaubas.2013.01.003
Karlinska E, Kaczorowska O, Romanowska O, et al. Nutritional and polyphenolic composition of Agrimonia procera Wallr. from experimental cultivation with different levels of nitrogen fertilization. Molecules. 2022;27(21):7597. https://doi.org/10.3390/molecules27217597.
Ciobanu N, Cojocaru-Toma M, Ciobanu C, Benea A. Evaluation of polyphenolic profile and antioxidant activity of some species cultivated in the Republic of Moldova. Eur J Anal Chem. 2019:441-447.
Khalaf H, El-Saadani R, El-Desouky A, Abdeldaiem M, Elmehy M. Antioxidant and antimicrobial activity of gamma-irradiated chicory (Cichorium intybus L.) leaves and roots. J Food Measure. 2018;12:1843-51. doi: 10.1007/s11694-018-9798-0.
Spina M, Cuccioloni M, Sparapani L, Acciarri S, Eleuteri A, Fioretti E, Angeletti M. Comparative evaluation of flavonoid content in assessing quality of wild and cultivated vegetables for human consumption. J Sci Food Agric. 2008;88:294-304. doi: 10.1002/jsfa.3089.
Nwafor IC, Shale K, Achilonu M. Chemical composition and nutritive benefits of chicory (Cichorium intybus) as an ideal complementary and/or alternative livestock feed supplement. Sci World J. 2017;2017:7343928. doi: 10.1155/2017/7343928.
Street R, Sidana J, Prinsloo G. Cichorium intybus: traditional uses, phytochemistry, pharmacology, and toxicology. Evid Based Complement Alternat Med. 2013;2013:1-13. doi: 10.1155/2013/579319.
Perović J, Tumbas Šaponjac V, Kojić J, Krulj J, Moreno D, García-Viguera C, Bodroža-Solarov M, Ilić N. Chicory (Cichorium intybus L.) as a food ingredient – nutritional composition, bioactivity, safety, and health claims: a review. Food Chem. 2021;336:127676. doi: 10.1016/j. foodchem.2020.127676.
Ohindovsci A, Cojocaru-Toma M, Calalb T, Ancuceanu R, et al. Extraction methods of polyphenols in aerial parts of Galium verum L. In: Industrial pharmacy – realities and prospets: Collection of International Scientific-Practical Conference. Kharkiv, Ukraina; 2022. p. 73-77.
Vlase L, Mocan A, Hanganu D, et al. Comparative study of polyphenolic content, antioxidant and antimicrobial activity of Galium species (Rubiaceae). Dig J Nanomater Biostruct. 2014;9(3):1085-1094.
Hanganu D, Burtescu R, Petrescu S, et al. Galium species - polyphenolic content and their antioxidant potential. Hop Med Plants. 2018;26(1-2):84-94.
Mitova M, Anchev M, Handjieva N, et al. Iridoid patterns in Galium L. and some phylogenetic considerations. Z Naturforsch C J Biosci. 2002;57(3-4):226-34. doi: 10.1515/znc-2002-3-405.
Lakic N, Mimica-Dukić N, Isak J, Božin B. Antioxidant properties of Galium verum L. (Rubiaceae) extracts. Centr Eur J Biol. 2010;5(3):331-7. doi: 10.2478/s11535-010-0022-4.
Layali I, Ebrahimzadeh M, Joulaei M. Antioxidant properties of Galium verum. Int J Life Sci Pharm Res. 2016;6(3):31-37.
Bradic J, Percovic A, Tomovic M. Phytochemical and pharmacological properties of some species of the genus Galium L. (Galium verum and mollugo). Serbian J Exp Clin Res. 2021;22(3):187-93. doi: 10.1515/sjecr-2017–0057.
Budzianowski J, Thiem B, Kikowska M. Solidago virgaurea L. - chemical composition, traditional and medicinal use, pharmaceutical properties, potential applications, and biotechnological studies-a review. In: Ekiert H, Ramawat K, Arora J, editors. Medicinal plants: domestication, biotechnology and regional importance. Cham: Springer; 2021. P. 661-692. https://doi.org/10.1007/978-3-030-74779-4_20.
Fursenco C, Calalb T, Uncu L, Dinu M, Ancuceanu R. Solidago virgaurea L.: a review of its ethnomedicinal uses, phytochemistry, and pharmacological activities. Biomolecules. 2020;10(12):1619. https://doi.org/10.3390/biom10121619
Apáti P, Szentmihályi K, Kristó S, Papp I, Vinkler P, Szoke É, Kéry Á. Herbal remedies of Solidago - correlation of phytochemical characteristics and antioxidative properties. J Pharm Biomed Anal. 2003;32(4-5):1045-53. doi: 10.1016/s0731-7085(03)00207-3.
Wozniak D, Slusarczyk S, Domaradzki K, Drys A, Matkowski A. Comparison of polyphenol profile and antimutagenic and antioxidant activities in two species used as source of Solidaginis herba - Goldenrod. Chem Biodivers. 2018;15(4):e1800023. doi: 10.1002/cbdv.201800023.
Gross S, Goodarzi G, Watabe M, Bandyopadhyay S, Pai S, Watabe K. Antineoplastic activity of Solidago virgaurea on prostatic tumor cells in an SCID mouse model. Nutr Cancer. 2002;43(1):76-81. doi: 10.1207/S15327914NC431_9.
Choi SZ, Choi SU, Lee K. Pytochemical constituents of the aerial parts from Solidago virga-aurea var. gigantea. Arch Pharm Res. 2004;27(2):164-168. doi: 10.1007/BF02980100.
Thiem B, Wesołowska M, Skrzypczak L, Budzianowski J. Phenolic compounds in two Solidago L. species from in vitro culture. Acta Pol Pharm. 2001;58(4):277-81.
Roslon W, Osinska E, Mazur K, Geszprych A. Chemical characteristics of European goldenrod (Solidago virgaurea L. subsp. virgaurea) from natural sites in central and Eastern Poland. Acta Sci Pol Hortorum Cultus. 2014;13(1):55-65.
Tamas M. Cercetări chemotaxonomice la genul Solidago [Chemotaxonomique researches in
Solidago species]. Contrib Bot (Cluj-Napoca). 1986:110-113.Dobjanschi L, Fritea L, Patay E, Tamas M. Comparative study of the morphological and phytochemical characterization of Romanian Solidago species. Pak J Pharm Sci. 2019;32(4):1571-9.
Kielkopf CL, Bauer W, Urbatsch IL. Bradford assay for determining protein concentration. Cold Spring Harb Protoc. 2020;4:102269. doi: 10.1101/pdb. prot102269.
Smith J. SDS polyacrylamide gel electrophoresis of proteins. Methods Mol Biol. 1984;1:41-5. doi: 10.1385/0-89603-062-8:41.
Nowakowski A,Wobig WJ, Petering DH. Native SDS-PAGE: high resolution electrophoretic separation of proteins with retention of native properties including bound metal ions. Metallomics, 2014;6(5):1068-78. doi: 10.1039/c4mt00033a.
Kruger N. The Bradford method for protein quantitation. In: Walker JM, editor. The protein protocols handbook. 2nd ed. Totowa: Humana Press; 2002. p. 17-24.
Walker J, editor. The protein protocols handbook. 2nd ed. Totowa: Humana Press; 2002. 1146 p.
Pace N, Treviño S, Prabhakaran E, Scholtz M. Protein structure, stability and solubility in water and other solvents. 2004;359(1448):1225-34, doi: 10.1098/rstb.2004.1500.