Introduction
A major role and at the same time a question mark, both for patients and doctors, is the possibility that drugs and anesthetic/analgesic techniques influence cancer metastasis. Cancer is the leading cause of death worldwide. This trend will continue in the future [1, 2]. Most of the deaths of cancer patients are due to complications arising from metastases. These may result from direct, lymphatic or hematogenous spread. The metastasis process of a tumor depends on its intrinsic properties and interaction with the host [3]. The treatment of tumors by performing a surgical intervention, radical or palliative, have a significant impact [4]. For these reasons, the rate of survival and migration of cancer cells in the perioperative period is studied quite insistently and complexly [5]. Thus, surgical intervention and anesthetic support in cancer patients becomes of great importance, because it represents the vulnerable link, both from the point of view of the operation itself, as well as the possibility that drugs, anesthetic techniques may or may not influence tumor metastasis [6, 7].
Material and methods
There were studied primary scientific studies published from 1996 to 2021 dedicated to loco-regional anesthesia/analgesia and its influence on the perioperative period and on cancer metastasis. To achieve the proposed goal, scientific sources PubMed, Google Scholar, Medscape, SCOPUS, MEDLINE were used. Keywords used for searching: loco-regional anesthesia, fascia plane anesthesia, metastasis. More than 80 reference sources were identified, and 67 were selected for analysis.
Results and discussions
After analyses of the sources, we can describe some of the principal mechanisms of metastasis that occur during the perioperative period. The surgical procedure, itself, performed for tumor resection – is a risk factor for metastasis by creating an environment with high potential for tumor cell survival [8]. Metastasis occurs when cancer cells succeed in suppressing the immune system (decreases the activity of natural kinase cells) [9]. Virtually, all perioperative antineoplastic treatment creates relative immunosuppression [10]:
manipulations on the tumor during surgery favor the penetration of its cells into the systemic circulation;
the presence of the primary tumor is an inhibitor of angiogenesis and its removal eliminates the defense mechanism;
perioperative immunosuppression, which primarily influences cellular immunity. A negative role is played by neuroendocrine and inflammatory components in response to stress, but also by the action of preparations administered during anesthesia and postoperative analgesia;
hypothermia can also be attributed to the suppression of immune function.
The physiological response to stress in surgery causes relative immunosuppression through the release of hormonal mediators: catecholamines, prostaglandins, and growth factors [11]. Prostaglandins and catecholamines can cause activation of receptors that increase the metastatic capacity of cancer cells (e.g., β2-adrenergic) [12] and cyclooxygenase-2 receptors [13]. Inflammation associated with tissue trauma results in the release of cytokines (interleukin-6 and prostaglandin E2) that can cause inhibition of natural killer (NK) cell activity [14]. The role of NK cells is essential in the perioperative phase as they are responsible for the detection and destruction of circulating tumor cells [15].
Another factor contributing to cancer recurrence is tissue hypoxia. This causes an increase in the expression of transcription factor 1-alpha (HIF1A), which plays an important role in promoting cellular pathways for angiogenesis, cell proliferation, and metastasis [16]. The mechanism of action of HIF1A is to determine the expression of vascular endothelial growth factor (VEGF) [17]. This, in turn, stimulates tumor growth and angiogenesis, which can remodel lymphatic pathways, allowing metastasis of tumor cells [18, 19].
Hemotransfusion is associated with increased risk of metastasis [20]. Transfused blood induces immunosuppression. There are scientific data that show decreased natural killer cells, T-helper cells, and likewise decreased cytokine production [21]. The term immunomodulation induced by hemotransfusion was introduced in 1973. It is also associated with increased risk of cancer recurrence in patients given blood components preoperatively [22]. Hemotransfusion is associated with increased risk of metastasis [20]. Transfused blood induces immunosuppression. There is scientific evidence of decreased natural killer cells, T-helper cells and decreased cytokine production [21]. The term immunomodulation induced by hemotransfusion has been proposed in 1973. This is also associated with increased risk of cancer recurrence in patients who are transfused blood components perioperatively [22]. Perioperative hypothermia is a factor that increases the risk of wound infection. It is considered, that maintaining a normal temperature in a patient during the perioperative period is more effective, than antibiotic prophylaxis. Hypothermia during general anesthesia inhibits cellular immunity, especially activity of natural killer cells, thus increasing cancer recurrence [23]. For example, intraoperative decrease of body temperature down to 35.5 ºС, in patients who underwent abdominal surgery, has a the immunosuppressive effect[24].
Effects of anesthesia
Clinical trials on the effects of anesthetics on cancer are complicated to conduct because patients need a combination of anesthetic agents. It would be medically and ethically difficult to perform surgery without perioperative pain relief e.g., using only regional anesthesia. Usually, intraoperative analgesia includes both regional analgesic and general anesthetic components. In the following paragraphs, we describe some comparative studies between local and general anesthesia on cancer metastasis. These have been performed on patients with tumors form different location who underwent surgery.
The first large randomized trial in this field for breast cancer surgery and was published in 2019 on approximately 2100 patients who underwent mastectomy or local surgery with axillary dissection, and were randomly assigned to receive regional anesthesia/analgesia (paravertebral block combined with sedation) or general anesthesia (inhalation, opioid analgesia) [25]. Cancer recurrences were similar in both groups: general/combined anesthesia occurring in 10% of patients in each group during a 36-month follow-up. Another study that included more than 1,700 patients who underwent major abdominal or thoracic surgery and were similarly randomized: epidural + general anesthesia versus general anesthesia + postoperative opioids. Thus, overall survival and recurrence-free survival were similar in the two groups at more than five years of follow-up [26]. In a randomized trial including 400 patients with lung cancer who underwent video-assisted thoracoscopic surgery, we have the following results: relapse-free survival and overall survival were similar in both patients receiving general anesthesia + postoperative opioid analgesia and those receiving general anesthesia + postoperative epidural analgesia [27]. In the next paragraphs, we will show the results from other scientific publications in which research has been described regarding the role of anesthetics in the development of metastases according to their mode of action.
General anesthesia (inhalation)
Laboratory studies have suggested some possible mechanism by which inhalational anesthetics may promote metastasis [28-31]. Inhalational anesthetic agents (e.g., isoflurane, sevoflurane, desflurane, and halothane) also have pro-inflammatory effects [32].
General (intravenous) anesthesia
According to multiple researchers that studied the effects of anesthetics on natural killer (NK) cell activity and metastasis development in rats modelled with breast cancer, propofol did not suppress NK cell activity nor did metastases develop, while halothane, ketamine and thiopental both decreased NK cell activity and metastases developed [33].
Regional anesthesia/analgesia
Regional anestheisa/analgesia could reduce cancer recurrence through several mechanisms, for example:
decreasing the stress response to surgery (by pain control or sympathetic blockade) [34, 35];
reducing the need for opioids or inhalants;
via direct effects related to absorption of local anesthetics.
This type of anesthesia/analgesia is the most intensely discussed by the medical community because of the prospects for their use in general surgery as well as in oncology. Thus, we will point to studies that have been performed on large groups of patients.
The first large international randomized trials were conducted comparing regional anesthesia + propofol and general anesthesia with sevoflurane + opioids. It found similar relapse rates after surgery for breast cancer [25]. The same results were obtainted in two other studies describing epidural versus intravenous general anesthesia + opioids [26, 27]. In 2014, more than 3,000 people were included in studies and underwent cancer surgery. According to these data, no difference in cancer recurrence or overall survival was observed in patients given general anesthesia + epidural and general anesthesia alone [36]. Subsequently in 2017 were conducted 28 studies (retrospective, observational and randomized) including more than 67,000 patients who underwent surgery for multiple cancers. Overall and relapse-free survival was the same in those who received regional anesthesia with or without general anesthesia [37]. Ten other retrospective studies including approximately 13,760 individuals, after radical prostatectomy, with a diagnosis of prostate cancer were also reviewed. According to the results of these studies, regional anesthesia with or without general anesthesia, was described with better overall survival, but similar cancer recurrences with general anesthesia alone [38]. In conjunction with follow-up studies, investigations of the immune status of breast cancer patients treated with surgery given propofol + paravertebral regional anesthesia and inhaled general anesthesia + opioids was performed. Thus, it has been shown that in patients receiving regional and intravenous anesthesia in breast cancer tissue there is both increased infiltration with immune cells, increased apoptosis of cancer cells and maintenance of NK cell cytotoxicity [29, 30, 39].
Amide local anesthetics, particularly lidocaine, have been used for a long time in pain management during general anesthesia, as well as systemic intravenous infusions, in neuraxial and peripheral nerve blocks. Lidocaine is a short-acting substance with minimal toxicity. It blocks voltage-dependent sodium channels, which are responsible for the generation of impulses in sensory nerve endings and conduction of pain impulses through nerve fibers. [40-42] Along with its analgesic effect, lidocaine also exhibits antioncogenic and anti-inflammatory properties through various pathways [43-47]. In general, local anesthetics, according to some studies, have antitumor and antimetastatic properties. These effects are achieved through several mechanisms, such as:
direct cytotoxicity, induction of apoptosis;
inhibition of proliferation, migration and invasion;
modulation of gene expression through methylation [48].
Local anesthetics (LA) in high concentrations are known to be cytotoxic to neuronal cells. This seems to be correlated with the lipid solubility of LA. As a result, this process includes cell death by necrosis or apoptosis. All local anesthetics cause necrosis, and lidocaine and bupivacaine cause apoptosis in neuroblastoma cells [49, 50] as well as in breast and thyroid cancer cells [51, 52]. Apoptosis is controlled by an intracellular cysteine group – caspases. Chang and colleagues demonstrated that treatment of breast cancer cells with clinically relevant concentrations of lidocaine and bupivacaine induced caspase formation [51]. As a result, cell viability decreased and the process of apoptosis was triggered [53, 54]. Another article studied the action of lidocaine and bupivacaine on thyroid cancer cells, the latter induced apoptosis. This effect was mediated via the protein kinase pathway [51]. Local anesthetics also inhibit proliferation, migration and invasion of cancer cells. Yoon and colleagues introduced tetracaine and lidocaine into the tumor region. This has been shown to inhibit microtubule expansion and the ability of tumor cells to promote aggregation and reattachment [55]. LAs may also influence proliferation and invasion through their effects on cell signaling pathways [56, 57].
Several studies have been carried out on this issue:
Mammamoto demonstrated that lidocaine, in clinically relevant concentrations, decreased the invasiveness of HT1080 cells by inhibiting HB-EGF excretion [58];
Sakaguchi showed that lidocaine inhibited EGF-induced proliferation of tongue cancer cells [59];
Piegeler demonstrated that amide local anesthetics reduced TNF-α-induced Src activation and ICAM-1 phosphorylation in human lung cancer cells and inhibited cancer cells [60];
Baptista-Hon and colleagues demonstrated that ropivacaine inhibiting sodium channels decreases cell invasion. This is due to direct effects on cancer cells [61], but also indirect effects by blocking noxious stimuli [62].
Local anesthetics also inhibit cancer cell proliferation by modulating gene expression through DNA methylation. Lidocaine has been shown to demethylate DNA in breast cancer cells [63].
Since, cancer treatment is mainly surgical, used anesthesia must also be appropriate, meeting certain criteria. In general, this needs to be effective, safe and with as lowest risk of early and late postoperative complications as possible. To achieve this goal, the anesthesiologist has several methods in his arsenal: strong general anesthetics, effective analgesics both opioid and non-opioid, different types of loco-regional anesthesia/analgesia. Thus, nowadays, the radical method of treatment in oncology is considered surgery. However, its late results are insufficient, because the percentage of tumor recurrence is considerable. Cancer metastases are the cause of death in 90% of cases [64]. It is necessary for anesthetists working in cancer centers to differentiate between anesthesia/analgesia methods and their effects on cancer recurrence. Thus, research conducted by Ovechkin A. M. in 2012 at the First Moscow State Medical University I. M. Sechenov, showed us in major lines the anesthetic/analgesic remedies that influence the immune status of the oncological patient (Table 1, 2) [65].
Table 1. Remedies with suppressive effect on immune system during the perioperative period [65]. | |
Drug | Potential action on anti-cancer immunity |
Ketamine | Decreases the amount and activity of natural killer cells. |
Thiopental | Decreases the amount and activity of natural killer cells. |
Midazolam | Decreases the plasma concentration of IL-8 cytokines. This favors immunosuppression, because IL-8 is a factor that activates neutrophil chemotaxis and adhesion (important components for the normal immune response to surgical aggression). |
Inhalation anesthetics | In the experiment, it inhibits interferon stimulation of natural killer cells. Sevoflurane in vitro decreases the clearance of tumor necrosis factor by natural killer cells. Decreased long-term results in melanoma interventions under inhalation anesthesia compared to regional anesthesia are demonstrated. |
Nitrous oxide | In experiments, it causes the appearance and accelerates the formation of metastases in the lungs and liver. It is the most powerful stimulator of the formation of metastases in the liver among all the anesthetic preparations studied |
Morphine | In the experiment it inhibits cellular immunity and the activity of natural killer cells |
Fentanyl | Decreases the amount and activity of natural killer cells in clinic |
α2-adrenoreceptor agonists (clonidine) | It accelerates cell proliferation and inhibits apoptosis. In the experiment, it favors the progression of mammary gland tumor growth |
Note: IL-8 - Interleukin 8. | |
Table 2. Remedies with positive effect on immune system during the perioperative period. [65] | |
Drug | Potential action on anti-cancer immunity |
Propofol | It has an immunoprotective effect, decreases the metastatic potential of a line of cancer cells, induces the process of apoptosis, increases the synthesis of anti-inflammatory cytokines IL-10 |
Local Anesthetics | Lidocaine inhibits the activity of the receptors of the endothelial growth factor and the proliferation of tumor cells (in vitro). Ropivacain inhibits the growth of tumor cells (in vitro) |
Tramadol | In the experiment and in the clinic it stimulates the activity of natural killer cells; it does not allow the metastasis of the tumor which is induced by the surgical intervention (experimental data) |
Nonsteroidal anti-inflammatory drugs | In the experiment, the negative action on angiogenesis and tumor growth is demonstrated, induce apoptosis, balance the negative action of morphine on the immune status |
Blockers of β-adrenoreceptors | In the experiment it inhibits tumor growth, which is determined by β-adrenergic stimulation
|
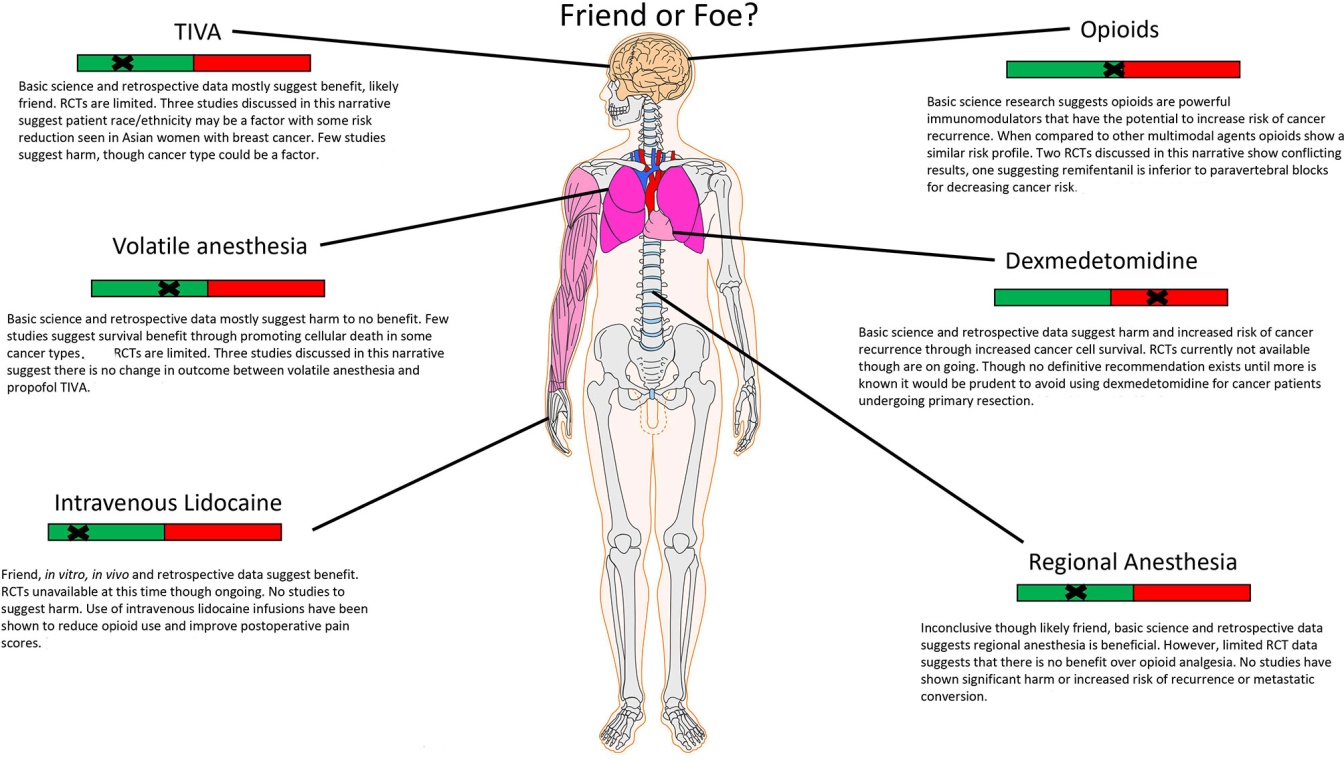
Julio Montejano and Vesna Jevtovic-Todorovic in 2021 wrote an article: Anesthesia and cancer, Friend or foe? A narrative analysis. In the article, they exemplified the types of anesthesia and their interaction with cancer (Fig. 1) [66].
|
Fig. 1 Anesthesia and Cancer, Friend or Foe? |
Conclusions
Surgical treatment in cancer patients is associated with a multitude of factors that directly or indirectly may influence tumor cell survival, inflammatory and stress responses to surgical aggression. Inhalational anesthetics have pro-inflammatory effects and may influence cancer cell survival, including immune suppression and up-regulation of hypoxia-inducible factors (HIF1A). However, experiments on animal and human regarding cancer recurrence after the use of inhalational agents were conflicting. Propofol has anti-inflammatory and antioxidant effects that protect against immune suppression and may preserve natural killer (NK) cell activity. Clinical trials comparing intravenous and inhaled anesthetic agents have shown mixed results in terms of cancer recurrence. Regional anesthesia could decrease cancer recurrence by reducing the need for opioids or inhaled anesthetics, or by reducing the stress response during surgery. Other studies suggest that opioids might influence metastasis or tumor growth, however, the evidence is conflicting and inconclusive. There is in vitro scientific evidence of a protective effect of systemic lidocaine on recurrent cancer, although relevant clinical data are limited.
Competing interests
None declared
Authors' contribution
All authors contributed equally to the drafting and writing of the manuscript. The authors read and approved the final version of the manuscript.
Authors’ ORCID IDs
Ruslan Baltaga – https://orcid.org/0000-0003-0659-4877
Andrei Perciun – https://orcid.org/0000-0003-0401-0604
Radu Turchin – https://orcid.org/0000-0002-3509-1219
Valeria Cotelea – https://orcid.org/0000-0001-9379-7642
References
Sekandarzad MW, van Zundert AAJ, Doornebal CW, Hollmann MW. Regional anesthesia and analgesia in cancer care: is it time to break the bad news? Curr Opin Anaesthesiol. 2017;Oct;30(5):606-612. doi: 10.1097/ACO.0000000000000492.
Forget P, Aguirre JA, Bencic I, Borgeat A, Cama A, Condron C, Eintrei C, Eroles P, Gupta A, Hales TG, Ionescu D, Johnson M, Kabata P, Kirac I, Ma D, Mokini Z, Guerrero Orriach JL, Retsky M, Sandrucci S, Siekmann W, Štefančić L, Votta-Vellis G, Connolly C, Buggy D. How anesthetic, analgesic and other non-surgical techniques during cancer surgery might affect postoperative oncologic outcomes: a summary of current state of evidence. Cancers (Basel). 2019 Apr 28;11(5):592. doi: 10.3390/cancers11050592.
Langley RR, Fidler IJ. The seed and soil hypothesis revisited - the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer. 2011 Jun 1;128(11):2527-35. doi: 10.1002/ijc.26031.
Badan A. Optimizarea asistenţei preoperatorii la bolnavele de cancer mamar supuse radioterapiei şi chimioterapiei [Optimizing preoperative care for breast cancer patients undergoing radiotherapy and chemotherapy]. Info-Med (Chisinau). 2008;(2/14):36-39. Romanian.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002 Dec 19;420(6917):860-7. doi: 10.1038/nature01322.
Global Burden of Disease Cancer Collaboration; Fitzmaurice C, Abate D, Abbasi N, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5(12):1749-1768. doi: 10.1001/jamaoncol.2019.2996.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015 Mar 1;136(5):E359-86. doi: 10.1002/ijc.29210.
Engilbertsson H, Aaltonen KE, Björnsson S., Kristmundsson T., Patschan O., Rydén L., Gudjonsson S. Transurethral bladder tumor resection can cause seeding of cancer cells into the bloodstream. J. Urol., 2015 Jan;193(1):53-7. doi: 10.1016/j.juro.2014.06.083.
Brittenden J, Heys SD, Ross J, Eremin O. Natural killer cells and cancer. Cancer. 1996 Apr 1;77(7):1226-43. doi: 10.1002/(sici)1097-0142(19960401)77:7<1226::aid-cncr2>3.0.co;2-g.
Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth. 2010;105(2):106-115. https://doi.org/10.1093/bja/aeq164.
Alazawi W, Pirmadjid N, Lahiri R, Bhattacharya S. Inflammatory and immune responses to surgery and their clinical impact. Ann Surg. 2016 Jul;264(1):73-80. doi: 10.1097/SLA.0000000000001691.
Pérez-Sayáns M, Somoza-Martín JM, Barros-Angueira F, Diz PG, Gándara Rey JM, García-García A. Beta-adrenergic receptors in cancer: therapeutic implications. Oncol Res. 2010;19(1):45-54. doi: 10.3727/096504010x12828372551867.
Wang Z, Chen JQ, Liu JL. COX-2 inhibitors and gastric cancer. Gastroenterol Res Pract. 2014;2014:132320. doi: 10.1155/2014/132320.
Angka L, Khan ST, Kilgour MK, Xu R., Kennedy MA, Auer RC. Dysfunctional natural killer cells in the aftermath of cancer surgery. Int J Mol Sci. 2017 Aug 17;18(8):1787. doi: 10.3390/ijms18081787.
Tavare AN, Perry NJ, Benzonana LL, Takata M, Ma D. Cancer recurrence after surgery: direct and indirect effects of anesthetic agents. Int J Cancer. 2012 Mar 15;130(6):1237-50. doi: 10.1002/ijc.26448.
Huang H, Benzonana LL, Zhao H, Watts HR, Perry NJ, Bevan C, Brown R, Ma D. Prostate cancer cell malignancy via modulation of HIF-1α pathway with isoflurane and propofol alone and in combination. Br J Cancer. 2014 Sep 23;111(7):1338-49. doi: 10.1038/bjc.2014.426.
Darby IA, Hewitson TD. Hypoxia in tissue repair and fibrosis. Cell Tissue Res. 2016 Sep;365(3):553-62. doi: 10.1007/s00441-016-2461-3.
Schito L, Semenza GL. Hypoxia-inducible factors: master regulators of cancer progression. Trends Cancer. 2016 Dec; 2(12):758-770. doi: 10.1016/j.trecan.2016.10.016.
Karnezis T, Shayan R, Caesar C, Roufail S, Harris NC, Ardipradja K, Zhang YF, Williams SP, Farnsworth RH, Chai MG, Rupasinghe TW, Tull DL, Baldwin ME, Sloan EK, Fox SB, Achen MG, Stacker SA. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell. 2012 Feb 14;21(2):181-95. doi: 10.1016/j.ccr.2011.12.026.
Weber RS, Jabbour N, Martin RC 2nd. Anemia and transfusions in patients undergoing surgery for cancer. Ann Surg Oncol. 2008 Jan;15(1):34-45. doi: 10.1245/s10434-007-9502-9.
Chen G, Zhang FJ, Gong M, Yan M. Effect of perioperative autologous versus allogeneic blood transfusion on the immune system in gastric cancer patients. J Zhejiang Univ Sci B. 2007 Aug;8(8):560-5. doi: 10.1631/jzus.2007.B0560.
Amato A, Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev. 2006 Jan 25;2006(1):CD005033. doi: 10.1002/14651858.CD005033.pub2.
Ben-Eliyahu S, Shakhar G, Rosenne E, Levinson Y, Beilin B. Hypothermia in barbiturate-anesthetized rats suppresses natural killer cell activity and compromises resistance to tumor metastasis: a role for adrenergic mechanisms. Anesthesiology. 1999 Sep;91(3):732-40. doi: 10.1097/00000542-199909000-00026.
Beilin B, Shavit Y, Razumovsky J, Wolloch Y, Zeidel A, Bessler H. Effects of mild perioperative hypothermia on cellular immune responses. Anesthesiology. 1998 Nov;89(5):1133-40. doi: 10.1097/00000542-199811000-00013.
Sessler DI, Pei L, Huang Y, Fleischmann E, Marhofer P, Kurz A, Mayers DB, Meyer-Treschan TA, Grady M, Tan EY, Ayad S, Mascha EJ, Buggy DJ; Breast Cancer Recurrence Collaboration. Recurrence of breast cancer after regional or general anaesthesia: a randomised controlled trial. Lancet. 2019 Nov 16;394(10211):1807-1815. doi: 10.1016/S0140-6736(19)32313-X.
Du YT, Li YW, Zhao BJ, Guo XY, Feng Y, Zuo MZ, Fu C, Zhou WJ, Li HJ, Liu YF, Cheng T, Mu DL, Zeng Y, Liu PF, Li Y, An HY, Zhu SN, Li XY, Li HJ, Wu YF, Wang DX, Sessler DI; Peking University Clinical Research Program Study Group. Long-term survival after combined epidural-general anesthesia or general anesthesia alone: follow-up of a randomized trial. Anesthesiology. 2021 Aug 1;135(2):233-245. doi: 10.1097/ALN.0000000000003835.
Xu ZZ, Li HJ, Li MH, Huang SM, Li X, Liu QH, Li J, Li XY, Wang DX, Sessler DI. Epidural anesthesia-analgesia and recurrence-free survival after lung cancer surgery: a randomized trial. Anesthesiology. 2021 Sep 1;135(3):419-432. doi: 10.1097/ALN.0000000000003873.
Benzonana LL, Perry NJ, Watts HR, Yang B, Perry IA, Coombes C, Takata M, Ma D. Isoflurane, a commonly used volatile anesthetic, enhances renal cancer growth and malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology. 2013 Sep;119(3):593-605. doi: 10.1097/ALN.0b013e31829e47fd.
Buckley A, McQuaid S, Johnson P, Buggy DJ. Effect of anaesthetic technique on the natural killer cell anti-tumour activity of serum from women undergoing breast cancer surgery: a pilot study. Br J Anaesth. 2014 Jul;113 Suppl 1:i56-62. doi: 10.1093/bja/aeu200.
Desmond F, McCormack J, Mulligan N, Stokes M, Buggy DJ. Effect of anaesthetic technique on immune cell infiltration in breast cancer: a follow-up pilot analysis of a prospective, randomised, investigator-masked study. Anticancer Res. 2015 Mar;35(3):1311-9.
Ecimovic P, McHugh B, Murray D, Doran P, Buggy DJ. Effects of sevoflurane on breast cancer cell function in vitro. Anticancer Res. 2013 Oct;33(10):4255-60.
Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth. 2008;22(3):263-77. doi: 10.1007/s00540-008-0626-2.
Melamed R, Bar-Yosef S, Shakhar G, Shakhar K, Ben-Eliyahu S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg. 2003 Nov;97(5):1331-1339. doi: 10.1213/01.ANE.0000082995.44040.07.
Hahnenkamp K, Herroeder S, Hollmann MW. Regional anaesthesia, local anaesthetics and the surgical stress response. Best Pract Res Clin Anaesthesiol. 2004 Sep;18(3):509-27. doi: 10.1016/j.bpa.2004.01.004.
O'Riain SC, Buggy DJ, Kerin MJ, Watson RWG, Moriarty DC. Inhibition of the stress response to breast cancer surgery by regional anesthesia and analgesia does not affect vascular endothelial growth factor and prostaglandin E2. Anesth Analg. 2005 Jan;100(1):244-249. doi: 10.1213/01.ANE.0000143336.37946.7D.
Pei L, Tan G, Wang L, Guo W, Xiao B, Gao X, Wang L, Li H, Xu Z, Zhang X, Zhao J, Yi J, Huang Y. Comparison of combined general-epidural anesthesia with general anesthesia effects on survival and cancer recurrence: a meta-analysis of retrospective and prospective studies. PLoS One. 2014 Dec 30;9(12):e114667. doi: 10.1371/journal.pone.0114667.
Grandhi RK, Lee S, Abd-Elsayed A. The relationship between regional anesthesia and cancer: a metaanalysis. Ochsner J. 2017 Winter;17(4):345-361.
Lee BM, Singh Ghotra V, Karam JA, Hernandez M, Pratt G, Cata JP. Regional anesthesia/analgesia and the risk of cancer recurrence and mortality after prostatectomy: a meta-analysis. Pain Manag. 2015 Sep;5(5):387-95. doi: 10.2217/pmt.15.30.
Jaura AI, Flood G, Gallagher HC, Buggy DJ. Differential effects of serum from patients administered distinct anaesthetic techniques on apoptosis in breast cancer cells in vitro: a pilot study. Br J Anaesth. 2014 Jul;113 Suppl 1:i63-7. doi: 10.1093/bja/aet581.
Kutay Yazici K, Kaya M, Aksu B, Ünver S. The effect of perioperative lidocaine infusion on postoperative pain and postsurgical recovery parameters in gynecologic cancer surgery. Clin J Pain. 2021 Feb 1;37(2):126-132. doi: 10.1097/AJP.0000000000000900.
Weibel S, Jelting Y, Pace NL, Helf A, Eberhart LH, Hahnenkamp K, Hollmann MW, Poepping DM, Schnabel A, Kranke P. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database Syst Rev. 2018 Jun 4;6(6):CD009642. doi: 10.1002/14651858.CD009642.pub3.
Chu R, Umukoro N, Greer T, Roberts J, Adekoya P, Odonkor CA, Hagedorn JM, Olatoye D, Urits I, Orhurhu MS, Umukoro P, Viswanath O, Hasoon J, Kaye AD, Orhurhu V. Intravenous lidocaine infusion for the management of early postoperative pain: a comprehensive review of controlled trials. Psychopharmacol Bull. 2020 Oct 15;50(4 Suppl 1):216-259.
Khan JS, Hodgson N, Choi S, Reid S, Paul JE, Hong NJL, Holloway C, Busse JW, Gilron I, Buckley DN, McGillion M, Clarke H, Katz J, Mackey S, Avram R, Pohl K, Rao-Melacini P, Devereaux PJ. Perioperative pregabalin and intraoperative lidocaine infusion to reduce persistent neuropathic pain after breast cancer surgery: a multicenter, factorial, randomized, controlled pilot trial. J Pain. 2019 Aug;20(8):980-993. doi: 10.1016/j.jpain.2019.02.010.
Lee JT, Sanderson CR, Xuan W, Agar M. Lidocaine for cancer pain in adults: a systematic review and meta-analysis. J Palliat Med. 2019 Mar;22(3):326-334. doi: 10.1089/jpm.2018.0257.
Hermanns H, Hollmann MW, Stevens MF, Lirk P, Brandenburger T, Piegeler T, Werdehausen R. Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: a narrative review. Br J Anaesth. 2019 Sep;123(3):335-349. doi: 10.1016/j.bja.2019.06.014.
Galoș EV, Tat TF, Popa R, Efrimescu CI, Finnerty D, Buggy DJ, Ionescu DC, Mihu CM. Neutrophil extracellular trapping and angiogenesis biomarkers after intravenous or inhalation anaesthesia with or without intravenous lidocaine for breast cancer surgery: a prospective, randomised trial. Br J Anaesth. 2020 Nov;125(5):712-721. doi: 10.1016/j.bja.2020.05.003.
Van Haren F, van den Heuvel S, Radema S, van Erp N, van den Bersselaar L, Vissers K, Steegers M. Intravenous lidocaine affects oxaliplatin pharmacokinetics in simultaneous infusion. J Oncol Pharm Pract. 2020 Dec;26(8):1850-1856. doi: 10.1177/1078155220905011.
Xuan W, Hankin J, Zhao H, Yao S, Ma D. The potential benefits of the use of regional anesthesia in cancer patients. Int J Cancer. 2015 Dec 15;137(12):2774-84. doi: 10.1002/ijc.29306.
Perez-Castro R, Patel S, Garavito-Aguilar ZV, Rosenberg A, Recio-Pinto E, Zhang J, Blanck TJ, Xu F. Cytotoxicity of local anesthetics in human neuronal cells. Anesth Analg. 2009 Mar;108(3):997-1007. doi: 10.1213/ane.0b013e31819385e1.
Werdehausen R, Fazeli S, Braun S, Hermanns H, Essmann F, Hollmann MW, Bauer I, Stevens MF. Apoptosis induction by different local anaesthetics in a neuroblastoma cell line. Br J Anaesth. 2009 Nov;103(5):711-8. doi: 10.1093/bja/aep236.
Chang YC, Liu CL, Chen MJ, Hsu YW, Chen SN, Lin CH, Chen CM, Yang FM, Hu MC. Local anesthetics induce apoptosis in human breast tumor cells. Anesth Analg. 2014 Jan;118(1):116-24. doi: 10.1213/ANE.0b013e3182a94479.
Chang YC, Hsu YC, Liu CL, Huang SY, Hu MC, et al. Local anesthetics induce apoptosis in human thyroid cancer cells through the mitogen-activated protein kinase pathway. PLoS One. 2014;9(2):e89563. https://doi.org/10.1371/journal.pone.0089563.
Maehara Y, Kakeji Y, Kabashima A, Emi Y, Watanabe A, Akazawa K, Baba H, Kohnoe S, Sugimachi K. Role of transforming growth factor-beta 1 in invasion and metastasis in gastric carcinoma. J Clin Oncol. 1999 Feb;17(2):607-14. doi: 10.1200/JCO.1999.17.2.607.
Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012 Jan 1;188(1):21-8. doi: 10.4049/jimmunol.1101029.
Yoon JR, Whipple RA, Balzer EM, Cho EH, Matrone MA, Peckham M, Martin SS. Local anesthetics inhibit kinesin motility and microtentacle protrusions in human epithelial and breast tumor cells. Breast Cancer Res Treat. 2011 Oct;129(3):691-701. doi: 10.1007/s10549-010-1239-7.
Mammoto T, Higashiyama S, Mukai M, Mammoto A, Ayaki M, Mashimo T, Hayashi Y, Kishi Y, Nakamura H, Akedo H. Infiltration anesthetic lidocaine inhibits cancer cell invasion by modulating ectodomain shedding of heparin-binding epidermal growth factor-like growth factor (HB-EGF). J Cell Physiol. 2002 Sep;192(3):351-8. doi: 10.1002/jcp.10145.
Hirata M, Sakaguchi M, Mochida C, Sotozono C, Kageyama K, Kuroda Y, Hirose M. Lidocaine inhibits tyrosine kinase activity of the epidermal growth factor receptor and suppresses proliferation of corneal epithelial cells. Anesthesiology. 2004 May;100(5):1206-10. doi: 10.1097/00000542-200405000-00024.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002 Dec 19-26;420(6917):860-7. doi: 10.1038/nature01322.
Sakaguchi M, Kuroda Y, Hirose M. The antiproliferative effect of lidocaine on human tongue cancer cells with inhibition of the activity of epidermal growth factor receptor. Anesth Analg. 2006 Apr;102(4):1103-7. doi: 10.1213/01.ane.0000198330.84341.35.
Piegeler T, Votta-Velis EG, Liu G, Place AT, Schwartz DE, Beck-Schimmer B, Minshall RD, Borgeat A. Antimetastatic potential of amide-linked local anesthetics: inhibition of lung adenocarcinoma cell migration and inflammatory Src signaling independent of sodium channel blockade. Anesthesiology. 2012 Sep;117(3):548-59. doi: 10.1097/ALN.0b013e3182661977.
Baptista-Hon DT, Robertson FM, Robertson GB, Owen SJ, Rogers GW, Lydon EL, Lee NH, Hales TG. Potent inhibition by ropivacaine of metastatic colon cancer SW620 cell invasion and NaV1.5 channel function. Br J Anaesth. 2014 Jul;113 Suppl 1:i39-i48. doi: 10.1093/bja/aeu104.
Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013 Dec;13(12):871-82. doi: 10.1038/nrc3627.
Lirk P, Berger R, Hollmann MW, Fiegl H. Lidocaine time- and dose-dependently demethylates deoxyribonucleic acid in breast cancer cell lines in vitro. Br J Anaesth. 2012 Aug;109(2):200-7. doi: 10.1093/bja/aes128. Erratum in: Br J Anaesth. 2013 Jan;110(1):165.
Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006 Nov 17;127(4):679-95. doi: 10.1016/j.cell.2006.11.001.
Ovechkin AM. Klinicheskaia patophiziologiia i anatomiia ostroi boli [Clinical pathophysiology and anatomy of acute pain]. Reg Anesth Acute Pain Manag J (Moscow). 2012;6(1):32-40. Russian.
Montejano J, Jevtovic-Todorovic V. Anesthesia and cancer, friend or foe? A narrative review. Front Oncol. 2021 Dec 23;11:803266. doi: 10.3389/fonc.2021.803266.