Introduction
While it's not clear why there has been a rise in complications related to gout, many experts believe that this may be due to a growing prevalence of factors that contribute to gout, such as higher rates of obesity and metabolic syndrome [1, 2]. Lifestyle factors have an important effect on the incidence of gout. This was confirmed by researchers, authors of a 12-year cohort study conducted on 47150 cases of gout. The risk of gout was more frequent among individuals who were either obese or had a greater consumption of alcohol overall, or in situations where both factors were present [3-5].
Tophus is a cardinal feature of gout; it is a complex mass consisting of monosodium urate crystals, a variety of immune and inflammatory cells, and a fibrous capsule [6, 7]. Tissue deposition of uric acid crystals initiates the formation of tophus with a local inflammatory response of fibrotic tissue. Clinically, it is difficult to separate the different components of a tophus, and these can vary between different anatomical sites in different patients. Stimulation of immune/inflammatory system cells through MUS crystals can lead to chronic inflammation, pain, and tissue remodeling, including bone erosions [8].
In 1998, the World Health Organization (WHO) described „metabolic syndrome” for the first time. The classification criteria for metabolic syndrome (MS) have been proposed by WHO, the National Adult Cholesterol Education Program Treatment Panel III (NCEP ATP III) and the International Diabetes Federation. However, the definition of MS is not yet consistent. Multiple studies have been conducted in recent decades about the effect of increased UA on MS development. Large epidemiological studies on the association between HU and MS showed that increased concentration of serum urea is often observed in subjects with MS. Scientists evaluated data from 8 669 participants in the third National Health and Nutrition Review Study (NHANES-III) (1988-1994) and demonstrated that MS prevalence increased with increasing serum UA levels [9-11].
In another study, out of a total of 2 374 subjects who received a health exam, subjects with HU had a 1.63 times higher risk of MS compared to those without HU based on MS criteria defined by the American Heart Association/National Institute of Heart, Lungs and Blood. In two other studies, the average level of serum UA in patients with MS was approximately 0.5-1.0 mg/dL higher than controls. In addition, another study showed that serum UA levels increased with the number of components of MS after adjusting for age, sex, creatinine clearance, and the use of alcohol/diuretic. Recently, HU is recognized as a distinct feature and/or major associated factor of MS [12-15].
The aim of this study was to characterize of specific dysmetabolic changes and features of extraarticular evolution in patients with gout.
Material and methods
A descriptive study was conducted, and 501 patients with gout were included. There were 423 males and 78 females in the study. The mean age of gout debut was 49.2 (36.9; 59.9). The study was carried out in accordance with the requirements of the Ministry of Health for „Clinical and financial-economic research” within the postdoctoral scientific program at the Nicolae Testemiţanu State University of Medicine and Pharmacy of the Republic of Moldova, Department of Internal Medicine, Discipline of rheumatology and nephrology. The patients were separated into two groups, depending on their age when gout debuted: ≤59 years (group I, 233 subjects) and the age of onset ≥60 years (group II, 268 subjects).
From the electronic medical records of the Departments of Arthrology, Rheumatology and Nephrology of the Republican Clinical Hospital „Timofei Moşneaga” were extracted the clinical, laboratory and treatment data points on 693 patients with gout hospitalized between 2015-2022. Of the 693 patients, 501 met the including criteria and were selected for the statistical processing. The diagnosis of gout in the database was carried out in accordance with the classification criteria for gout according to ACR/EULAR 2015 [12]. The raw data was processed in SPSS version 26.0.
Results
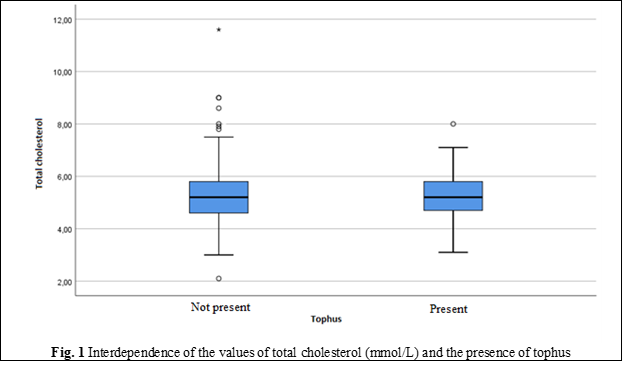
Metabolic syndrome - a complex of metabolic, hormonal, and clinical disorders associated with atherosclerosis, is detected in most of our patients with gout, but in a higher frequency among those with chronic tophaceous gout. Thus, our study showed that the frequency of MS in patients with tophaceous gout is 1.6 – 2.8 times higher than in the population, reaching 65% in people over 60 years of age. The main components of MS currently include abdominal obesity, impaired lipid (Table 1, Figures 1-5) and carbohydrates metabolism (Figure 6), hypertension and insulin resistance. The frequency of detection of individual components of MS in patients with gout is also quite high and made it possible to diagnose MS in 68% of cases, insulin resistance in – 67%, diabetes mellitus type 2 – in 18% and hypertension – in almost 80%.
The analysis of our study included results from 268 patients who were >60 years, had a chronic course of gouty arthritis and poor drug control. Tophi, a classical characteristic of chronic gout, was present in 27.6% cases (74 patients), and absent in 72.4% cases (194 patients).
Thus, according to our data, in patients with tophaceous gout, the severity of obesity and LDL-cholesterol (including cholesterol) was higher, while HDL-cholesterol was lower, causing an increase in the frequency of high blood pressure and type 2 diabetes, with age differences in the frequency of detection of MS and insulin resistance. The independent association between insulin resistance and MS prevalence is confirmed by many studies [16]. Therefore, the data from the literature, have noted that serum levels of UA correlated with the risk of developing both MS and its components – obesity, hypertension, and dyslipidemia, but inversely correlated with hyperglycemia (Figure 1) [17, 18].
The prevalence of MS in patients with gouty chronic arthritis was 30%-42% according to NCEP ATP III guidelines and 50%-59% according to WHO obesity criteria, both of which were significantly higher than normal control groups. These findings showed a concomitant increase of prevalence of MS and gout and suggested that the two diseases are related [19-22].
Table 1. Values of the basic components of metabolic syndrome | |||
| Tophus | ||
Not present | Present | ||
Total cholesterol | Minimum, Maximum | 2.10; 72.60 | 3.10; 8.00 |
Mean | 5.42 | 5.21 | |
Standard Deviation | 3.71 | 0.89 | |
Median | 5.20 | 5.20 | |
Percentile 25 | 4.60 | 4.70 | |
Percentile 75 | 5.80 | 5.80 | |
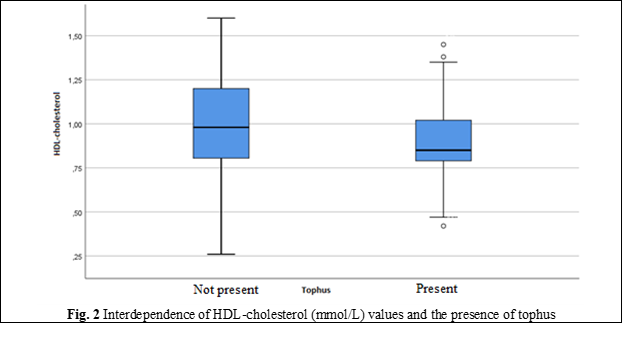
HDL-cholesterol | Minimum, Maximum | 0.26; 1.60 | 0.42; 1.45 |
Mean | 1.00 | 0.91 | |
Standard Deviation | 0.25 | 0.20 | |
Median | 0.98 | 0.85 | |
Percentile 25 | 0.81 | 0.79 | |
Percentile 75 | 1.20 | 1.02 | |
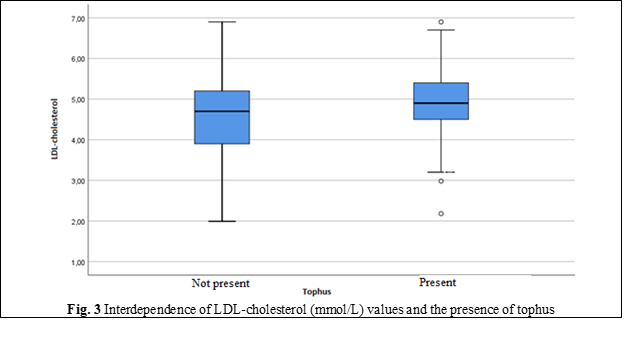
LDL-cholesterol | Minimum, Maximum | 1.99; 6.90 | 2.18; 6.90 |
Mean | 4.60 | 4.91 | |
Standard Deviation | 0.87 | .86 | |
Median | 4.70 | 4.90 | |
Percentile 25 | 3.90 | 4.50 | |
Percentile 75 | 5.20 | 5.40 | |
Triglycerides | Minimum, Maximum | 0.47; 5.90 | 0.80; 12.40 |
Mean | 2.48 | 2.52 | |
Standard Deviation | 0.99 | 1.22 | |
Median | 2.10 | 2.20 | |
Percentile 25 | 1.80 | 1.90 | |
Percentile 75 | 3.30 | 2.70 | |
Uric acid in serum | Minimum, Maximum | 81.00; 747.00 | 160.00; 797.00 |
Mean | 432.70 | 480.02 | |
Standard Deviation | 119.96 | 117.64 | |
Median | 437.00 | 480.00 | |
Percentile 25 | 345.00 | 418.30 | |
Percentile 75 | 506.50 | 559.00 | |
Hemoglobin A1C | Minimum, Maximum | 0.90; 58.30 | 0.40; 10.50 |
Mean | 6.29 | 5.99 | |
Standard Deviation | 3.83 | 1.18 | |
Median | 5.90 | 6.00 | |
Percentile 25 | 5.20 | 5.30 | |
Percentile 75 | 6.80 | 6.40 | |
Note: HDL-cholesterol – high-density lipoprotein; LDL-cholesterol – low-density lipoprotein. | |||
The values of the main clinical and laboratory indicators of blood (glucose, UA, creatinine) exceeded the normal and showed significant differences in groups with predominance in those with chronic tophaceous gout. Indicators of lipid metabolism also had significant differences in groups: cholesterol, TG, LDL-C levels were higher, and HDL-cholesterol was below the optimal values in those with tophi. Hyperuricemia was detected in 2/3 of the patients in both groups (65% and 66%).
It was found that five metabolic risk factors have significant or moderately significant correlations with the UA levels in the general group, as demonstrated in Figures 1-6.
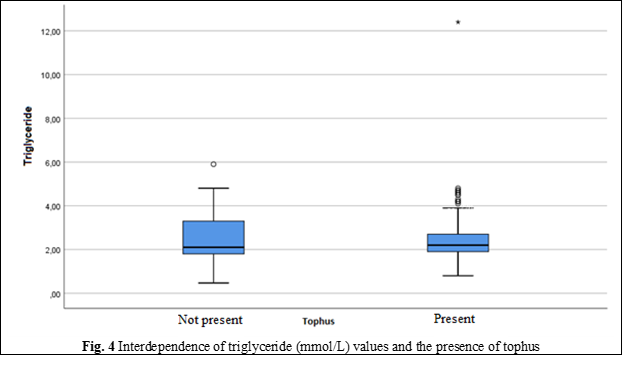
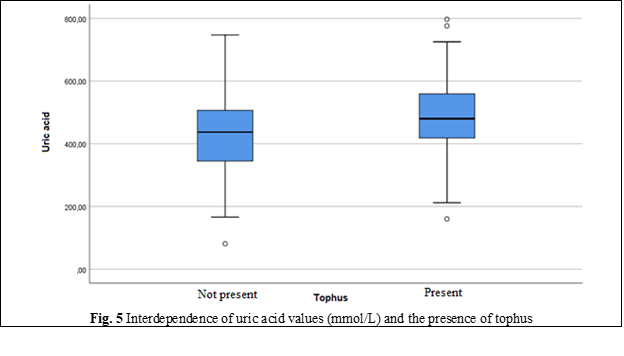
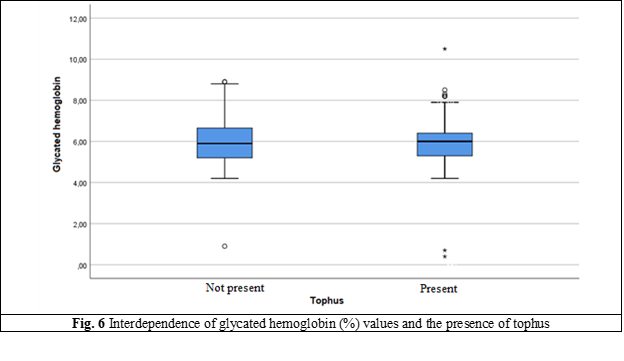
In people without metabolic syndrome, uric acid levels did not exceed the upper reference value, except for fasting blood glucose (p = 0.146). In people with metabolic syndrome, it was found that the levels of glucose, triglycerides, HDL and waist circumference have a slight or moderate correlation, while blood pressure did not give such a correlation with uric acid [21]. The effectiveness of non-pharmacological measures to reduce the level of hyperlipidemia is confirmed by reducing the values of total cholesterol and LDL-cholesterol, but it does not have a significant rate.
Discussions
The prevalence of MS and hyperuricemia/gout has steadily increased. There are data on the epidemiology of gout and hyperuricemia in US adults in NHANES-III (1988-1994) and NHANES 2007-2008 that show that the prevalence of these conditions is substantial and has increased over the past two decades. In addition, the prevalence of MS has been shown to steadily increase according to the data from NHANES-III and NHANES 1999-2006 in the US population. Similarly, in the Korean population over the age of 20, scientists have demonstrated that MS’s prevalence has gradually increased from 24.9% in 1998 to 31.3% in 2007. Several clinical studies showed a higher prevalence of MS in patients with gout compared to the general population [22].
In 2022, a few researchers studied the relationship of metabolic syndrome and gout, for an average of 7.4±1.2 years. 88 058 men with gout were examined. The incidence of gout was 3.36 per 1000 people. It has been found that gout in people with MS develops 2 times more often. Of all the components of MS, abdominal obesity came out on the first place; increased TG was in second place [23]. In a large cross-sectional study conducted in NHANES-III, the prevalence of MS in people with gout was 62.8% vs. 25.4% among people without gout according to age and gender [24].
An analysis carried out in the ARIC study provides similar data for women. A total of 6263 women between the ages of 45 and 64, with no history of gout before inclusion in the study, were tracked for 9 years, identifying 106 cases of incident gout. Compared to women with a BMI <25 kg/m2 at the baseline, the adjusted relative risk of gout was 1.63. In women with BMI 25–29.9 kg/m2 and BMI ≥30 kg/m2 at age 25 years, multivariate relative risks for gout were 3.36 and 2.84, respectively, compared to those with BMI <25 kg/m2 at age 25 years. Women with the highest weight gain indicator (≥16.3 kg) from the age of 25 to the initial age had a 2 times higher risk of gout compared to people in the lowest indicator [25].
In the case study of the THIN database, persons with a BMI between 25-29 kg/m2 had relative risk of 1.62 for gout and those with BMI of ≥30 kg/m2, a relative risk of 2.34. With a BMI of 21-22.9 kg/m2, the relative risk of gout in men was RR 0.85 in those with BMI <21 kg/m2, increasing to a relative risk of 1.40 in those with BMI 23-24.9 kg/m2, RR 1.95 with BMI 25-29.9 kg/m2, RR 2.33 with BMI 30-34.9 kg/m2 and RR 2.97 with BMI ≥ 35 kg/m2 [25]. In addition, the risk of the incidence of gout was increased in men who added 20-29 kg and ≥30 kg in weight from the age of 21, compared to those whose weight was stable. In contrast, weight loss of 10 kg or more reduced the risk of gout by 39% [26].
Although hyperglycemia and IR are recognized components of MS, the role of DM type 2 as a risk factor for the development of gout has received relatively little attention. Interestingly, in the case study THIN database-control, people with DM type 2 had a 33% lower risk of developing gout than those without DM type 2 (RR 0.67) [5, 19, 27]. This finding was more marked in men than in women. The risk of developing gout reduced with increased duration DM type 2: duration 0-3 years RR 0.81, 4-9 years RR 0.67, and 10 years or more RR 0.52. The risk was also lower for DM type 1 than for DM type2. Although these findings may seem counter-intuitive, the predisposition to HU and gout induced by hyperinsulinemia and IR in the pre-diabetic state is considered to be reversed by the uricosuric effects of glycosuria once DM type 2 complications develop [2, 17, 28].
Thus, the incidence of metabolic syndrome is 2 times higher in people with tophaceous gout compared without it (33.6% and 15.9%, respectively, after adjusting according to age, p < 0.001). This indicator increases with age, and after 60 years, MS occurs in 47.6% of those suffering from gout, which is 2 times more common than in people who do not suffer from gout of the same age (23.8%). Since MS increases the risk of atherosclerotic cardiovascular disease and DM type 2, its presence significantly aggravates the comorbid background, complicates treatment and worsens the prognosis for gout [23, 28].
Conclusions
Gout is associated with a severe lipid metabolism dysfunction, significantly increasing the rate of metabolic syndrome especially among patients with chronic tophaceous gout, than in the group of patients with gout under the age of 59 years. On the other hand, in patients with the onset of gout who are ≤59 years lipid metabolism disorders occur significantly earlier than in patients with the onset of gout at the age of ≥60 years (p < 0.05).
Abbreviations
BMI – body mass index, DM – diabetes mellitus, HU – hyperuricemia, IR – insulin resistance, RR – relative risk; MS – metabolic syndrome, MUS – mono-urate of sodium, NCEP ATP III – National Adult Cholesterol Education Program Treatment Panel III, NHANES – National Health and Nutrition Review Study, UA – uric acid.
Competing interests
None declared
Author’s ORCID ID
Larisa Rotaru – https://orcid.org/0000-0002-3260-3426
References
Ali N, Miah R, Hasan M, et al. Association between serum uric acid and metabolic syndrome: a cross-sectional study in Bangladeshi adults. Sci Rep. 2020 May 12;10(1):7841. doi: 10.1038/s41598-020-64884-7.
Kleinman NL, Brook RA, Patel PA, et al. The impact of gout on work absence and productivity. Value Health. 2007 Jul-Aug;10(4):231-7. doi: 10.1111/j.1524-4733.2007.00173.x. PMID: 17645677.
Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet. 2016 Oct 22;388(10055):2039-2052. doi: 10.1016/S0140-6736(16)00346-9.
Richette P, Bardin T. Gout. Lancet. 2010 Jan 23;375(9711):318-28. doi: 10.1016/S0140-6736(09)60883-7.
Dalbeth N, Choi HK, Joosten LA, et al. Gout. Nat Rev Dis Primers. 2019 Sep 26;5(1):69. doi: 10.1038/s41572-019-0115-y.
Masseoud D, Rott K, Liu-Bryan R, Agudelo C. Overview of hyperuricaemia and gout. Curr Pharm Des. 2005;11(32):4117-24. doi: 10.2174/138161205774913318.
Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015 Nov;11(11):649-62. doi: 10.1038/nrrheum.2015.91.
de Oliveira EP, Burini RC. High plasma uric acid concentration: causes and consequences. Diabetol Metab Syndr. 2012 Apr 4;4:12. doi: 10.1186/1758-5996-4-12.
Harrold LR, Yood RA, Mikuls TR, et al. Sex differences in gout epidemiology: evaluation and treatment. Ann Rheum Dis. 2006 Oct;65(10):1368-72. doi: 10.1136/ard.2006.051649.
Mikuls TR, Farrar JT, Bilker W, et al. Gout epidemiology: results from the UK General Practice Research Database, 1990-1999. Ann Rheum Dis. 2005 Feb;64(2):267-72. doi: 10.1136/ard.2004.024091.
Trifirò G, Morabito P, Cavagna L, Ferrajolo C, Pecchioli S, Simonetti M, Bianchini E, Medea G, Cricelli C, Caputi AP, Mazzaglia G. Epidemiology of gout and hyperuricaemia in Italy during the years 2005-2009: a nationwide population-based study. Ann Rheum Dis. 2013 May;72(5):694-700. doi: 10.1136/annrheumdis-2011-201254.
Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med. 1987 Mar;82(3):421-6. doi: 10.1016/0002-9343(87)90441-4.
Lin KC, Lin HY, Chou P. The interaction between uric acid level and other risk factors on the development of gout among asymptomatic hyperuricemic men in a prospective study. J Rheumatol. 2000 Jun;27(6):1501-5.
Dalbeth N, Wong S, Gamble GD, et al. Acute effect of milk on serum urate concentrations: a randomised controlled crossover trial. Ann Rheum Dis. 2010 Sep;69(9):1677-82. doi: 10.1136/ard.2009.124230.
Thirunavukkarasu V, Anuradha CV. Influence of alpha-lipoic acid on lipid peroxidation and antioxidant defence system in blood of insulin-resistant rats. Diabetes Obes Metab. 2004 May;6(3):200-7. doi: 10.1111/j.1462-8902.2004.00332.x.
Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007 Feb 15;57(1):109-15. doi: 10.1002/art.22466.
Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005 Apr 11;165(7):742-8. doi: 10.1001/archinte.165.7.742.
Maynard JW, McAdams DeMarco MA, et al. Incident gout in women and association with obesity in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Med. 2012 Jul;125(7):717.e9-717.e17. doi: 10.1016/j.amjmed.2011.11.018.
Thottam GE, Krasnokutsky S, Pillinger MH. Gout and metabolic syndrome: a tangled web. Curr Rheumatol Rep. 2017 Aug 26;19(10):60. doi: 10.1007/s11926-017-0688-y.
Herman JB, Goldbourt U. Uric acid and diabetes: observations in a population study. Lancet. 1982 Jul 31;2(8292):240-3. doi: 10.1016/s0140-6736(82)90324-5.
Choi HK, Mount DB, Reginato AM, et al. Pathogenesis of gout. Ann Intern Med. 2005 Oct 4;143(7):499-516. doi: 10.7326/0003-4819-143-7-200510040-00009.
Choi HK, Soriano LC, Zhang Y, Rodríguez LA. Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case-control study. BMJ. 2012 Jan 12;344:d8190. doi: 10.1136/bmj.d8190.
Taylor WJ, Fransen, Jansen TL, et al. Study for updated gout classification criteria: identification of features to classify gout. Arthritis Care Res. (Hoboken). 2015 Sep;67(9):1304-1315. doi: 10.1002/acr.22585.
Rotaru L, Groppa L. La goutte et les comorbidites. Arch Balk Med Union. 2013:48(3 Suppl):307-309.
Rotaru L, Groppa L, Russu E, et al. Clinical and evolutive features of gouty arthritis. Arch Balk Med Union. 2022;57(4):356-362. https://doi.org/10.31688/ABMU.2022.57.4.04.
Rotaru L, Groppa L, Codreanu C, et al. Characteristics of clinical manifestations of gout in the elderly people. Rom J Rheumatol. 2022;31(3):118-123. doi: 10.37897/RJR.2022.3.3.
Rotaru L., Groppa L, Popa S, et al. Recovery of patients with gout. Mold J Health Sci. 2022;29(3):54-57. https://doi.org/10.52645/MJHS.2022.3.10.
Groppa L, Popa S, Rotaru L, et al. Reumatologie și nefrologie [Rheumatology and nephrology]. 2nd ed. Chisinau: Medicina; 2018. Romanian.