Introduction
Back pain has been called the „disease of the 21st century” because it has become a challenge for the public health system and is the leading cause of disability worldwide. Back pain is one of the dominant symptoms of ageing of the spine and affects up to 85% of the adult population at least once in their lifetime. Back pain is also one of the main causes of health care claims and sick leave in highly industrialized countries. Worldwide, back pain is the most common cause of work-related disability in people under 45 years of age and the third most common cause in people over 45 years of age.
In the 2010 and 2017 Global Burden of Disease studies, out of 291 pathologies analyzed, low back pain ranked as the largest contributor to global disability, being the leading cause of disability in 126 out of 195 countries.
Due to the intense process of demographic ageing, specific to the Republic of Moldova, the treatment of back pain is becoming a major priority for our medical system, which requires a systematic multidisciplinary approach.
The main causes of chronic back pain are the degenerative changes in the spine, such as yellow ligament and facet joints hypertrophy, the bulging of the posterior wall of the intervertebral disc, and marginal osteophyte formation, all of which lead to spinal canal stenosis, often associated with low-grade degenerative spondylolisthesis, which is an indicator of segmental instability of the spine. Lumbar spondylolisthesis is a condition of the spine involving the slippage of the upper vertebra in relation to the adjacent lower vertebra, resulting in narrowing of the neural foramina and compression of the emerging nerve roots.
With an annual incidence of 0.83% and a prevalence of 2.7% among men and 8.4% among women, we should now have around 15000 patients with lumbar degenerative spondylolisthesis in the Republic of Moldova, with a rate of 21612 new cases per year.
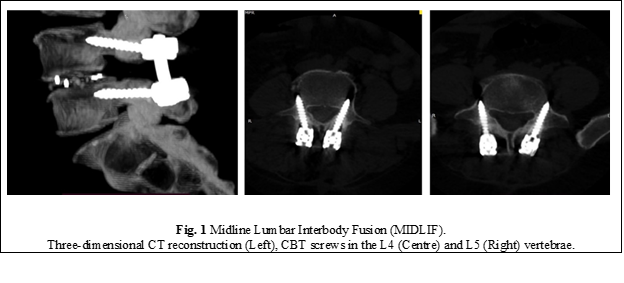
Currently, the standard intervertebral arthrodesis technique used in the treatment of degenerative spondylolisthesis involves osteosynthesis of the spine with transpedicular screws to enhance the success of bony fusion. The traditional techniques are very traumatic as they require extensive lateral dissection and tissue retraction to achieve an optimal screw placement angle, resulting in significantly increased operating time, considerable bleeding, increased morbidity, and a high rate of postoperative complications. To address some of these shortcomings of the traditional approach, the Midline Lumbar Interbody Fusion (MIDLIF®) technique has recently been developed. It involves the combination of the CBT screw fixation of the spine with intervertebral cage placement in order to achieve a solid interbody fusion (Fig. 1). The first report on this technique dates to 2009. Santoni et al. proposed the trajectory through the cortical bone and demonstrated that CBT screws have 30% higher pull-out strength resistance than traditional pedicle screws [1].
Subsequently, many morphometric and biomechanical studies have been carried out, demonstrating that CBT pedicle screws possess biomechanical properties equivalent to and sometimes superior to those of traditional pedicle screws [2-9]. However, the clinical efficacy of the MIDLIF technique in the treatment of low-grade degenerative spondylolisthesis is still unknown. All existing publications are studies with a low level of scientific evidence relevance, with most of them being observational, retrospective, or cohort studies. The only randomized clinical trial, published by Lee et al. in 2015 [10, 11], examines the efficacy of CBT screw fixation applied in a wide variety of degenerative spinal pathologies (canal stenosis with or without spondylolisthesis, degenerative disc disease, repeated herniated disc recurrences). To date, there have been no prospective, randomized, controlled clinical trials comparing the efficacy of the MIDLIF technique with that of traditional lumbar arthrodesis techniques (PLIF, TLIF) used exclusively in the treatment of degenerative spondylolisthesis.
To address this particular scientific problem, it was decided to conduct a randomized clinical trial to study the clinical efficacy and technical limitations of midline lumbar interbody fusion (MIDLIF) with CBT screws in order to develop an optimized algorithm for the assessment and treatment of patients with degenerative spondylolisthesis.
Materials and methods
A scientific and analytical study was carried out between 2017 and 2022 at the Department of Neurosurgery of the „Timofei Mosneaga” Republican Clinical Hospital, using the model of prospective and non-inferiority controlled clinical trials with randomized selection of group subjects. The study analysed the clinical and radiological effectiveness of the MIDLIF arthrodesis technique compared to the traditional lumbar interbody fusion techniques (TLIF or PLIF), used exclusively in the treatment of degenerative lumbar spondylolisthesis.
The scientific research project was favorably approved by the Research Ethics Committee of Nicolae Testemitanu SUMPh (minutes no.44 from December 12, 2016).
To achieve the planned objectives, after applying the inclusion and exclusion criteria, patients were assigned to two study groups:
Research group L1 included patients treated by the Midline Lumbar Interbody Fusion (MIDLIF) experimental technique;
Control group L0 was composed of patients treated by the traditional surgical techniques of PLIF or TLIF interbody fusion.
The sample size was estimated by applying the respective formula:
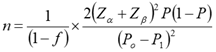
where:
P0 = proportion of patients in whom intervertebral fusion was achieved by the traditional method. According to the literature [12, 13], the success rate of achieving radiologic fusion in patients with spondylolisthesis using the traditional technique was 75.0% (P0 = 0.75);
P1 = the proportion of patients in the research group who had successful intervertebral fusion. We assume that the success rate of treatment after application of the new modified surgical technique will increase to 95.0% (P1 = 0.95);
P = (P0 + P1)/2 = 0.85;
Zα – tabular value. When “α” – significance threshold is 5%, then the coefficient Zα = 1.96;
Zβ – tabular value. When “β” – the statistical power of comparison is 80.0%, then the coefficient Zβ = 0.84;
f = the expected study dropout rate for various reasons q = 1/(1-f), f = 10.0% (0.1).
Entering the data into the formula, we obtained:
Therefore, two groups were created for the research: the L1 research group, which included no less than 56 patients with degenerative spondylolisthesis to whom the experimental surgical technique was applied, and the L0 control group, which included no less than 56 patients with degenerative spondylolisthesis to whom the traditional surgical technique was applied.
The randomization rate between the research groups was 1:1.
The study inclusion criteria were:
the presence of indications for surgical treatment by arthrodesis in cases of degenerative spinal disorders such as spondylosis associated with spondylolisthesis, foraminal stenosis, severe disc degeneration, and spinal degenerative instability;
low grade of spondylolisthesis (grade I-II);
age: 18 years and over;
the patient is competent to give informed consent;
acceptance to participate in the research.
The study exclusion criteria were:
spondylolisthesis with high degree of vertebral slippage (Meyerding grades III-V);
need for arthrodesis at 3 or more vertebral levels;
spinal canal stenosis of non-degenerative origin: tumor, trauma, etc.;
previous lumbar interbody fusion surgery;
active systemic or local infection;
severe spinal osteoporosis (DEXA T-score of -2.5 or less);
presence of contraindications for surgical treatment (such as severe medical co-morbidities, administration of immunosuppressive therapy, etc.);
lack of a permanent residence address in the Republic of Moldova, emigrants;
patient is unable to give voluntary consent;
patient refusal to participate in research.
The primary outcome measurement for assessing treatment efficacy was the successful fusion rate at 1 year postoperatively, as assessed by the presence of a continuous fusion mass either inside or outside the cage as seen on high-resolution, thin-slice three-dimensional computed tomography (CT) reconstructions.
To quantify the clinical effectiveness of the studied fusion techniques, data were collected prospectively from self-assessment questionnaires, which were completed by patients during the follow-up visits (preoperative, 1 month, 3 months, 6 months, and 1 year postoperatively).
The secondary outcome parameters included the intensity of low back pain and radiating pain, functional status, quality of life, and surgical morbidity. To assess the clinical effect on the improvement of the pain syndrome, the visual analogue pain scale (VAS) was used. Postoperative functional recovery was assessed using the Oswestry Disability Index (ODI) score questionnaire, which is a standard method of measuring the degree of disability associated with back pain. The influence of surgical treatment on health-related quality of life improvement was assessed by the SF-12 questionnaire. Surgical morbidity assessment included recording of operation time, incision length, estimated blood loss, need for blood transfusion, hospital stay, and encountered complications (dura mater lacerations, CSF leak, wound infections, pedicle and pars fractures, mechanical screw failures). Additionally, the degree of iatrogenic muscle injury was assessed by the increase in serum creatine kinase levels the first few days after surgery.
Results
The study enrolled 112 eligible patients with degenerative low-grade spondylolisthesis, randomly assigned into two groups. Patients were similar between groups considering demographic characteristics such as age, gender, body mass index, smoking status, comorbidities, and fused lumbar level (p > 0.05). The groups were also homogeneous preoperatively in terms of the VAS score for low back pain and radiating leg pain, the Oswestry Disability Index score, as well as the physical (PCS) and mental (MCS) components of the SF-12 score.
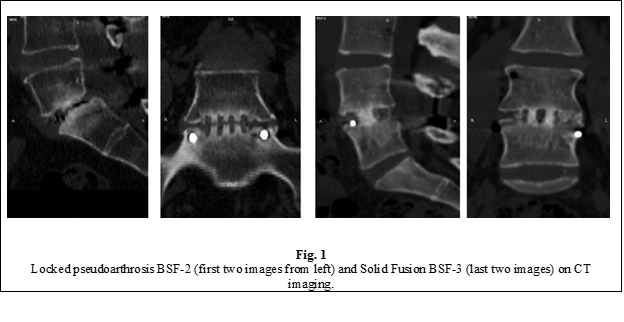
According to the CT scan, a solid interbody fusion (BSF grade 3) at one year after surgery was achieved in 47 patients (83.9%) in group L0 and 50 patients (89.3%) in group L1, with no significant difference between groups. A solid fusion through the vertebral plateaus associated with a horizontal area of radiolucency through the middle of the cage or intervertebral space (Fig. 2), described in the literature as "locked pseudoarthrosis", corresponding to a grade 2 fusion according to the Brantigan and Steffee (BSF) classification, was observed in 9 patients (16.1%) in the group L0 and 6 patients (10.7%) in the group L1. No cases of true pseudoarthrosis (BSF-1) were radiologically confirmed in either study group (Tab. 1). The difference in fusion rates between groups was not statistically significant, with the p-values for both the Pearson Chi-Square test (p = 0.405) and the Fisher exact test (p = 0.580) being much greater than 0.05.
Table 1. Success rate of interbody fusion. | |||
Fusion type | Brantigan and Steffee | L0 group | L1 group |
Radiographic pseudoarthrosis | BSF-1 | 0 | 0 |
Locked pseudoarthrosis | BSF-2 | 16.1% | 10.7% |
Radiographic fusion | BSF-3 | 83.9% | 89.3% |
Note: BSF – Brantigan and Steffee classification of pseudoarthrosis. | |||
The VAS score for low back pain at 1 year after surgery was significantly lower than the preoperative level in both groups, with the mean score decreasing from 7.18±2.22 pre-op to 3.48±1.57 at 1-year post-op in the L0 group and from 7.3±1.9 to 1.82±1.34 in the L1 group. The VAS score for back pain at 1-year post-op was significantly lower in the L1 group (p < 0.001). The VAS score for low back pain was also significantly lower in the L1 group compared to the L0 group at 1 month and 6 months postoperatively (p < 0.05), but this difference was not identified at 3 months post-op.
Similarly, the VAS score for radiating pain in the lower limbs improved significantly in both groups after surgery, with the mean score decreasing from 7.34±2.08 preoperatively to 2.27±1.61 at 1 year postoperatively in the L0 group and from 7.54±2.18 preoperatively to 0.73±1.29 at 1-year post-op. The difference between the groups at 1-year post-op was highly statistically significant (p < 0.001). Statistical analysis failed to identify a significant difference between the groups for the radiating pain VAS score at 1 month, 3 months, and 6 months post-op.
The Oswestry Disability Index score also improved significantly in both study groups after surgery, from 51.79±15.22 pre-op to 24.06±12.28 at 1-year post-op in the L0 group and from 46.45±15.77 to 11.51±8.66 in the L1 group, the difference being of strong statistical significance (p < 0.001). Also, a significant difference in ODI score between groups was found at 1 month (p < 0.001) and 6 months (p < 0.001), the functional improvement being more evident in the research group.
The improvement in health-related quality of life after treatment was assessed using the SF-12 score. The mental component summary (MCS) of the SF-12 score improved from 39.15±10.89 preoperatively to 51.05±9.2 at 1-year post-op in the L0 group and from 42.01±12.19 to 54.84±7.15 at 1-year post-op in the L1 group, the difference between groups being statistically significant (p < 0.05). There was also a significant difference between the study groups at 6 months postoperatively. However, no difference could be found between the groups at 1 month and 3 months post-op. At the same time, the physical component (PCS) of the SF-12 score improved from 27.15±7.33 pre-op to 37.41±8.09 in the L0 group and from 27.58±7.43 pre-op to 46.34±7.39 at 1-year postoperatively in the L1 group, the difference between groups being statistically significant (p < 0.001). The improvement of the physical component of the SF-12 score was significantly greater in the L1 group at 1, 3, and 6 months postoperatively (p < 0.05).
Regarding surgical morbidity, the L1 group was associated with better outcomes as compared to the L0 group in terms of intraoperative bleeding volume (p < 0.001), need for blood transfusions (p < 0.001), operative time (p < 0.05), incision length (p < 0.001), and increase in creatine kinase serum levels the first few days after surgery (p = 0.01). The general complication rate did not differ significantly between groups. Due to the neuronavigation guidance, there were no screw misplacements in either group. Mechanical problems such as pars or pedicle fracture due to screw placement, screw fracture or migration were not encountered in any patients in either group.
However, in both groups there were cases of inadvertent injury to the dura mater (5 cases in the control group and 4 cases in the study group), with one patient in the L1 group having postoperative CSF leakage through the wound and one patient in the L0 group having superficial wound infection. Both complications (CSF leakage, wound infection) were resolved without repeated surgery. One patient from each group developed adjacent level disease (at 5 years post-op in the group L1 and 2 years post-op in group L0). Both patients underwent minimally invasive decompression surgery without additional fusion.
Discussion
Lumbar fusion is widely used to treat lumbar spine disorders, including degenerative spondylolisthesis. Most of the spine fusions are performed with the aid of pedicle screw fixation because of the higher success rates. However, the use of pedicle screws has some important drawbacks. One is the need for a long skin incision and significant lateral muscle dissection due to the far lateral screw entry point. Pedicle screw fixation may lead to the risk of injuring the posterior medial branch of the spinal nerve, denervation of the paravertebral muscles, and superior facet joint violation, all of which lead to biomechanical failure and persistent lower back pain.
In 2009, Santoni et al. introduced a novel method of pedicle screw insertion known as the cortical bone trajectory (CBT), which follows a medial-to-lateral path in the transverse plane and a caudal-to-cephalad path in the sagittal plane through the pedicle. The purpose of the trajectory was to maximize the thread contact with the zone of higher bone density. Santoni found that CBT screws and traditional pedicle screws have equivalent pull-out strengths and toggle characteristics. CBT screws exhibit a 30% increase in uniaxial pull-out strength relative to pedicle screws [1].
Matsukawa et al. first highlighted in an in vivo study that the insertional torque of the CBT technique was higher than in the traditional pedicle technique.
The first comparison between CBT and traditional pedicle fixation for lumbar fusion was reported in 2015 by Lee et al. in a prospective randomized noninferiority trial [10]. Seventy-nine eligible patients were randomly assigned to either CBT or pedicle groups. The primary study endpoint was the fusion rate. Secondary end points included the intensity of lower back pain and pain radiating to the legs as measured by visual analogue scales, functional status improvement as measured by the ODI scale, surgical morbidity, and other outcomes such as mechanical screw failure and pedicle fractures. At the 12-month follow-up, similar fusion rates were observed in both groups. As for clinical outcome, CBT fixation provided similar improvements in pain and functional status. CBT fusion also resulted in a significantly shorter incision length, less blood loss, and a lower operative duration. Therefore, CBT screws in posterior lumbar interbody fusion provided similar clinical and radiologic outcomes compared with pedicle screws [14]. The study had some major limitations, like an insufficient sample size, a short follow-up period, and a broad spectrum of treated degenerative conditions.
Our study yielded several important findings. Similar fusion rates (>80%) were observed in both groups at the 12-month follow-up, with no significant statistical difference between the groups.
All BSF-2 arthrodesis cases were assessed by CT 2 years after surgery. None of the cases showed negative progression to true pseudoarthrosis. If we accept the statement that "locked pseudoarthrosis" (BSF-2) is a biomechanically stable construct that can be accepted as a satisfactory interbody fusion, we can consider that in both groups the success rate of arthrodesis was 100%.
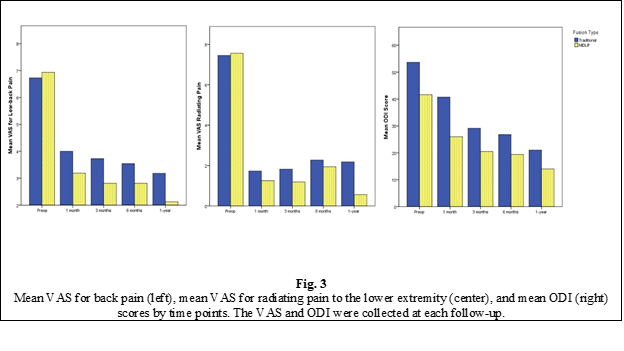
Clinically, both techniques have been very effective in relieving the back pain, the radiating pain, and the functional disability. However, MIDLIF provided a significantly better improvement in VAS of low back pain and radiating pain in the lower limb and a lower ODI score at 1 year post-operatively (Fig. 1), which is probably due to the minimally invasive nature of the experimental technique. In addition, MIDLIF resulted in lower surgical morbidity measured by incision length, operation time, blood loss and the need for blood transfusions, and the severity of muscle damage expressed by increased serum CK levels in the first postoperative days, compared to TLIF or PLIF. The reduction of surgery-related morbidity may be attributed to the medial insertion point and the medial-to-lateral trajectory of CBT screws, with shorter skin incisions and less lateral muscle dissection and retraction.
Our study partially confirms the results of the two other randomized controlled trials, which have demonstrated the efficacy of CBT screws in achieving a high fusion rate (>80%), lower surgical-related morbidity, and satisfactory clinical outcomes in the treatment of a broad spectrum of degenerative spinal pathologies [10, 15]. However, unlike our trial, the aforementioned studies found no difference between the groups in terms of back pain relief and functional recovery.
Conclusions
Based on the present study, we suggest that MIDLIF is a safe and effective technique for the treatment of degenerative spondylolisthesis. The MIDLIF technique provides the vertebral segment with adequate biomechanical stability, sufficient to achieve a solid interbody fusion. The success rate of intervertebral fusion in the MIDLIF technique is similar to that associated with traditional spondylolisthesis techniques. At the same time, MIDLIF offers all the specific benefits of a minimally invasive surgical technique, such as less postoperative pain, faster functional recovery, less bleeding, and fewer blood transfusions. Thus, MIDLIF might be a good alternative to the traditional fusion techniques in the treatment of degenerative low-grade spondylolisthesis.
Abbreviations
BSF – Brantigan and Steffee, CBT – cortical bone trajectory, MCS – mental component summary, MIDLIF – Midline Lumbar Interbody Fusion, PCS – physical component summary, PLIF – Posterior Lumbar Interbody Fusion, TLIF – Transforaminal Lumbar Interbody Fusion.
Competenig interests
None declared
Author’s ORCID ID
Serghei Borodin – https://orcid.org/0000-0003-2082-1976
References
Santoni BG, Hynes RA, McGilvray KC, Rodriguez-Canessa G, Lyons AS, Henson MA, et al. Cortical bone trajectory for lumbar pedicle screws. Spine J. 2009;9(5):366-73. doi: 10.1016/j.spinee.2008.07.008.
Matsukawa K, Taguchi E, Yato Y, Imabayashi H, Hosogane N, Asazuma T, et al. Evaluation of the fixation strength of pedicle screws using cortical bone trajectory: what is the ideal trajectory for optimal fixation? Spine. 2015;40(15):E873-8. doi: 10.1097/brs.0000000000000983.
Matsukawa K, Yato Y, Imabayashi H, Hosogane N, Abe Y, Asazuma T, et al. Biomechanical evaluation of fixation strength among different sizes of pedicle screws using the cortical bone trajectory: what is the ideal screw size for optimal fixation? Acta Neurochir (Wien). 2016;158(3):465-71. doi: 10.1007/s00701-016-2705-8.
Matsukawa K, Yato Y, Imabayashi H, Hosogane N, Asazuma T, Nemoto K. Biomechanical evaluation of the fixation strength of lumbar pedicle screws using cortical bone trajectory: a finite element study. J Neurosurg Spine. 2015;23(4):471-8. doi: 10.3171/2015.1.spine141103.
Matsukawa K, Yato Y, Kato T, Imabayashi H, Asazuma T, Nemoto K. In vivo analysis of insertional torque during pedicle screwing using cortical bone trajectory technique. Spine. 2014;39(4):E240-5. doi: 10.1097/brs.0000000000000116.
Rexiti P, Aierken G, Wang S, Abudurexiti T, Abuduwali N, Deng Q, et al. Anatomical research on strength of screw track fixation in novel cortical bone trajectory for osteoporosis lumbar spine. Am J Transl Res. 2019;11(11):6850-9.
Yu Y, Xie Y, Jian Q, Shi Y, Zhang G, Fan X. Biomechanical analysis and optimization of screw fixation technique for the cortical bone channel of lower thorax: study protocol clinical trial (SPIRIT Compliant). Medicine (Baltimore). 2020;99(7):e19046. doi: 10.1097/md.0000000000019046.
Nomoto EK, Fogel GR, Rasouli A, Bundy JV, Turner AW. Biomechanical analysis of cortical versus pedicle screw fixation stability in TLIF, PLIF, and XLIF applications. Global Spine J. 2019;9(2):162-8. doi: 10.1177/2192568218779991.
Perez-Orribo L, Kalb S, Reyes PM, Chang SW, Crawford NR. Biomechanics of lumbar cortical screw-rod fixation versus pedicle screw-rod fixation with and without interbody support. Spine. 2013;38(8):635-41. doi: 10.1097/BRS.0b013e318279a95e.
Lee GW, Son JH, Ahn MW, Kim HJ, Yeom JS. The comparison of pedicle screw and cortical screw in posterior lumbar interbody fusion: a prospective randomized noninferiority trial. Spine J. 2015;15(7):1519-26. doi: 10.1016/j.spinee.2015.02.038.
Lee GW, Ahn MW. Comparative study of cortical bone trajectory-pedicle screw (cortical screw) versus conventional pedicle screw in single-level posterior lumbar interbody fusion: a 2-year post hoc analysis from prospectively randomized data. World Neurosurg. 2018;109:e194-e202. doi: 10.1016/j.wneu.2017.09.137.
Shah RR, Mohammed S, Saifuddin A, Taylor BA. Comparison of plain radiographs with CT scan to evaluate interbody fusion following the use of titanium interbody cages and transpedicular instrumentation. Eur Spine J. 2003;12(4):378-85. doi: 10.1007/s00586-002-0517-4.
Choudhri TF, Mummaneni PV, Dhall SS, Eck JC, Groff MW, Ghogawala Z, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 4: radiographic assessment of fusion status. J Neurosurg Spine. 2014;21(1):23-30. doi: 10.3171/2014.4.spine14267.
Cofano F, Marengo N, Ajello M, Penner F, Mammi M, Petrone S, et al. The era of cortical bone trajectory screws in spine surgery: a qualitative review with rating of evidence. World Neurosurg. 2019;134:14-24. doi: 10.1016/j.wneu.2019.10.079.
Ding H, Hai Y, Liu Y, Guan L, Pan A, Zhang X, et al. Cortical trajectory fixation versus traditional pedicle-screw fixation in the treatment of lumbar degenerative patients with osteoporosis: a prospective randomized controlled trial. Clin Interv Aging. 2022;17:175-84. doi: 10.2147/cia.s349533.