Introduction
Parasitic arthritis is associated with infestation of the patient's body of parasitic species of worms and protozoan microorganisms. Now it has been established that parasitic arthritis can be caused by almost any species of these creatures. In most situations, they accompany such parasitic diseases as echinococcosis, taeniasis, schistosomiasis, filariasis, dracunculiasis, etc.
In recent years rheumatologists from all countries attest to a significant increase in the number of patients with parasitic arthritis [1]. According to specialized data, patients with parasitic arthritis constitute about 7% of the patients from rheumatology wards, 12% of patients develop a form of chronic peripheral arthritis and/or damage to the axial skeleton, which in 29% of cases cause disabilities [1, 2].
Parasitic arthritis can develop by two main mechanisms. The simplest of them is when the parasite itself or its eggs reach the joint cavity or surrounding tissues. The second mechanism is when lesions of the joint are caused by antibodies that are produced to fight the parasite located in other tissues or organs [3].
Echinococcosis is an anthropozoonosis that is usually asymptomatic in different mammals that are intermediate hosts for the parasite. Echinococcosis also affects humans, sometimes with a fatal outcome. Sensitization of the body by the products of parasitic metabolism leads to the development immediate hypersensitivity reaction and, after this, delayed type reaction of immunity. A vivid manifestation of an allergic reaction of an immediate type are eosinophilia and urticaria because of the outflow of echinococcal fluid, and in more severe cases (when opening the bladder) – anaphylactic shock. In the later stages of the disease, especially in multiple echinococcosis, immunopathological reactions play an important role. There are heterogenous clinical manifestations of echinococcosis and symptoms are determined by localization, size, multiplicity of invasion, rapid growth of the echinococcal cyst [4-8].
Another frequent form of helminthiasis widespread in our country is toxocariasis with a nematode causative agent of the Anisakidae family of the genus Toxocara. Several species of toxocariasis are known, but the most common are Toxocara canis, a helminth that mainly affects members of the canine family (dogs, wolves, foxes, arctic foxes, etc.) and Toxocara mystas, a helminth of the feline genus, sometimes called Toxocara cati. Clinical manifestations of toxocariasis are determined by the intensity of invasion, the distribution of larvae in organs and tissues, the frequency of re-inversion and the characteristics of the human immune response [5-7, 9]. There are several clinical forms of toxocariasis: (1) visceral; (2) cutaneous and (3) systemic.
Giardiasis is one of the most common protozoal diseases caused by Lamblia intestinalis (Giardia lamblia) [2, 4, 5, 9]. In the clinical manifestations, we can distinguish different lesions of the digestive tract (diarrhea, abdominal pain, vomiting, lack of appetite), hepatobiliary (dysfunction of the biliary tract), neurologic (irritability, fatigue, sleep disturbance, headache, dizziness), allergic manifestations, weight loss and anemia [3-5, 10, 11]. Hepatobiliary, cardiovascular, pulmonary, and musculoskeletal manifestations are common.
The purpose of the study was to research of the clinic and evolutional features of the cases of parasitic infections associated with damage of the osteo-articular system in helminthic pathologies.
Material and methods
In order to achieve the objectives, we have selected a group of 161 patients with the definite diagnosis of parasitic arthritis. The diagnosis was established in two stages – compliance according to the specific and serological criteria. The first stage included the diagnosis of inflammatory type of osteoarticular damage using the criteria. The second stage of diagnosis concerned the compliance with the positive results of the positive serological or parasitological diagnosis.
Patients were treated in the rheumatology and arthrology departments of the Timofei Moşneaga Republican Clinical Hospital and rheumatology department of the Saint Trinity Municipal Clinical Hospital, Chisinau, in the period 2017-2022 – according to the WMA Declaration of Helsinki with Favorable opinion of the Research Ethics Committee of Nicolae Testemițanu State University of Medicine and Pharmacy (No. 88 from 19.06.2018).
Patients were divided into 3 groups depending on the pathogen and the clinical variant of parasitic arthritis. The first group (97 patients) consisted of patients with parasitic arthritis associated with echinococcosis infestation, the 2nd (31 patients) – patients with parasitic arthritis associated with Toxocara cannis and the 3rd (33 patients) included patients with parasitic arthritis associated with Giardia lamblia infestation.
Criteria for inclusion in the study were age 18-70 years; the presence of parasitic infection; the appearance of symptoms of locomotor damage during a positive diagnosis of parasitic infection; damage to the musculoskeletal system for the first time (unsolved causes); patient's agreement to participate in the research. Exclusion criteria: history of rheumatic diseases (inflammatory, autoimmune); uncontrolled diseases (cardiovascular, hepatic, renal); neoplastic diseases; mental and cognitive disorders; <18 years or >70 years.
The index β-Tau was determined by applying the statistical method of trenchant analysis of homogeneous evolutionary data over time, with the calculation of the McNemar-Fisher index, which characterizes the rate of approach of the analyzed values to the ideal range (as an ideal interval it was considered the minimum and maximum value of the physiological norm). ANOVA's correlational factorial analysis established the degree of interaction between factors and the percentage share of different (independent) factors in the source of variation of another factor (dependent) [6-7]. This test has the advantage that it can compare the values of several batches at the same time, while the Student test cannot do so. The data were statistically processed in the software package STATISTICA 11.0.
Results
As can be seen from Table 1, the average age of the patients was 47.0±2.1 years, with the average debut of the disease 4.2±1.3 years, so only patients with chronic parasitic arthritis (the course approximately over 1 year) were included in the study. The joint function was classified according to the functional class (FC). FC stage I joint insufficiency was determined in 34.78% of patients, FC II – in 49.62% of patients, FC III – in 10.02% of persons and in 5.59% FC IV was determined. Sacroiliitis stage I (by Kellgren criteria (1963)), was diagnosed in 44 patients (27.32%), stage II – in 97 patients (59.46%), stage III – in 15 patients (10.1%) and stage IV in 5 (3.1%) patients. Recurrent bilateral conjunctivitis was detected in 97 (60.24%) patients. In 37 (22.98%) patients, oligoarthritis was detected, in 124 patients (77%) polyarthritis was present. Enthesopathy of the knee, hip, elbows, plants, spine was present in 141 (87.57%) patients, calcaneal spur 41 (25.46%) patients.
Table 1. General characteristic of the study batch. | ||
Indices assessed | Total patients (n = 161) | |
Age, years (Mean±median) | 47.0±2.1 | |
Sex M/F | 4/1 | |
Age of the disease, years (M±m) | 4.2±1.3 | |
Joint functional insufficiency (%) | Functional class I | 34.78±0.23* |
Functional class II | 49.62±0.35** | |
Functional class III | 10.02±0.16 | |
Functional class IV | 5.59±0.09 | |
Activity by score DAREA (%) | Degree I | 11.8±0.08 |
Degree II | 49.06±0.64** | |
Degree III | 39.13±0.71* | |
Unilateral sacroiliitis (%) | Stage I | 27.32±0.21* |
Stage II | 59.46±0.39** | |
Stage III | 10.1±0.07 | |
Stage IV | 3.1±0.09 | |
Note: *p < 0.05; **p < 0.01; DAREA – disease activity reactive arthritis score; M/F – male/female. | ||
All patients were divided into three groups, depending on the infestation agents detected. Parasitic arthritis is certain in the event that the appearance or aggravation of arthritis was preceded by an episode of helminthic invasion at least a month before. Parasitic arthritis is certain if it has developed one month after a diarrheal stool or an unstable stool and requires coprocytogram to exclude the impact of pathogenic intestinal flora [6-10].
Due to not compliance to the study protocol, or due to adverse effects developed during the study, 15 patients were excluded, and their data were not included in the final analysis.
In order to differentiate the general status of the patients according to the clinical and evolutive variant of the parasitic arthritis, the separation was performed according to etiological category of infestation agent, as presented in Table 2.
Table 2. Clinical-statutory parameters of the general study group, n = 161. | ||||
Clinical variant | Examined indexes | |||
Echinococcal form of parasitic arthritis (n = 97 patients) | Average age, years | 29.0±1.1 | ||
Sex M/F | 6/1 | |||
Age of the disease, years | 3.3±0.98 | |||
Joint functional incapacity (%) | FC I | 21.6 | ||
FC II | 51.2 | |||
FC III | 17.5 | |||
FC IV | 9.7 | |||
Activity, DAREA score (%) | Degree I | 15.9 | ||
Degree II | 63.8 | |||
Degree III | 20.3 | |||
Activity, ASDAS-CRP score (%) | <1.3 | 9.27 | ||
>1.3 < 2.1 | 36.08 | |||
>2.1 < 3.5 | 38.14 | |||
> 3.5 | 16.49 | |||
Unilateral sacroiliitis (%) | Stage I | 23.2 | ||
Stage II | 65.4 | |||
Stage III | 7.9 | |||
Stage IV | 3.5 | |||
Toxocariasis form of parasitic arthritis (n = 31 patients) | Average age, years | 33.0±3.4 | ||
Sex M/F | 1/2 | |||
Age of the disease, years | 2.4±0.56 | |||
Joint functional incapacity (%) | FC I | 27.9 | ||
FC II | 59.6 | |||
FC III | 10.2 | |||
FC IV | 2.3 | |||
Activity, DAREA score (%) | Degree I | 28.9 | ||
Degree II | 66.4 | |||
Degree III | 4.7 | |||
Activity, ASDAS-CRP score (%) | < 1.3 | 22.58 | ||
>1.3 < 2.1 | 61.29 | |||
>2.1 < 3.5 | 16.12 | |||
> 3.5 | 0 | |||
Unilateral sacroiliitis (%) | Stage I | 38.4 | ||
Stage II | 51.6 | |||
Stage III | 7.1 | |||
Stage IV | 1.93 | |||
Giardiasis form of parasitic arthritis (n = 33 patients) | Average age, years | 48.5±2.7 | ||
Sex M/F | 3/1 | |||
Age of the disease, years | 5.3±0.41 | |||
Joint functional incapacity (%) | FC I | 10.5 | ||
FC II | 51.2 | |||
FC III | 28.7 | |||
FC IV | 9.6 | |||
Activity, DAREA score (%) | Degree I | 8.7 | ||
Degree II | 59.8 | |||
Degree III | 31.5 | |||
Activity, ASDAS-CRP score (%) | < 1.3 | 0 | ||
>1.3 < 2.1 | 54.54 | |||
>2.1 < 3.5 | 36.36 | |||
> 3.5 | 9.09 | |||
Unilateral sacroiliitis (%) | Stage I | 14.5 | ||
Stage II | 48.9 | |||
Stage III | 31.6 | |||
Stage IV | 5.0 | |||
Note: CRP – C-reactive protein; DAREA – disease activity reactive arthritis score; M/F – male/female; ASDAS - Ankylosing Spondylitis Disease Activity Score. | ||||
Thus, reactive parasitic arthritis is characterized by a diversity of clinical joint manifestations, expressing itself through different clinical variants.
The spectrum of agents identified with clinical expression is presented as follows: Pulmonary echinococcosis was detected in 67 cases (41.61%), hepatic echinococcosis in 30 cases (18.63%), T. canis – in 31 cases (19.25%), Giardia lamblia – in 19 (11.8%), Giardia lamblia limited form (only joint syndrome predominates) in 14 cases (8.69%) (Table 3).
Table 3. Frequency of various parasitic forms in patients with parasitic arthritis. | ||
Pathogen and expression | No of patients n = 161 | % |
Pulmonary echinococcosis | 67 | 41.61 |
Hepatic echinococcosis | 30 | 18.63 |
Toxocara canis unfolded form | 31 | 19.25 |
Giardia lamblia unfolded form | 19 | 11.8 |
Giardia lamblia limited form (only joint syndrome predominates) | 14 | 8.69 |
The pathogens of the infestation have been coprologically and serologically diagnosed according to the corresponding National Clinical Protocols and the diagnosis has been established based on the clinical picture, which is possible when the main clinical-paraclinical criteria of inflammatory arthritis and the confirmation of parasitosis are used, in the absence of other possible rheumatic diseases.
The results of the analysis of the frequency of detection of parasitic agents depending on the type of clinical expression e of the joint syndrome, is presented in Figure 1.
|
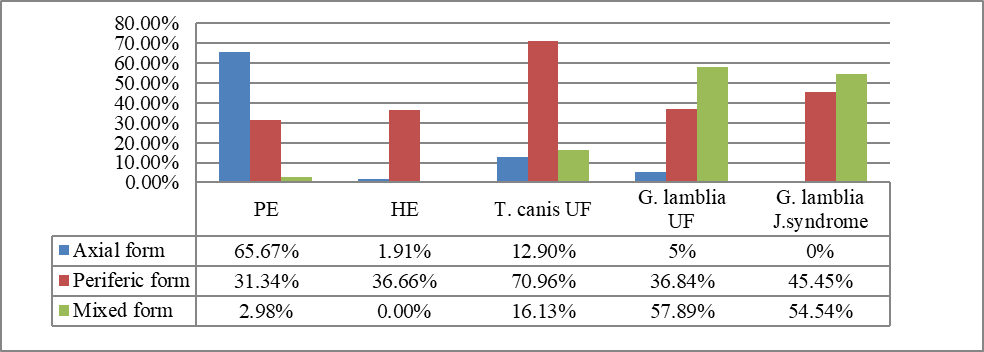
Fig. 1 The frequency of parasitic species presented depending on the clinical variant of the joint syndrome. Note: PE – pulmonary echinococcosis; HE – hepatic echinococcosis; T. canis UF – T. canis unfolded form; G. lamblia UF – Giardia lamblia unfolded form; G. lamblia J.syndrome – Giardia lamblia limited form (only joint syndrome predominates). |
Pulmonary echinococcosis was more often manifested by the axial (65.67%) and peripheral (31.34%) clinical forms, the form of mixed clinical expression being extremely rare (2.98%) (p < 0.001).The power of connection with the type of arthritis has reached the degree of statistical significance (Kendall-Tau index = 0.92; p < 0.001).
Hepatic echinococcosis was similar to the as the pulmonary echinococcosis, with the predominance of the axial variant (63.33%), followed by the peripheral one (36.66%) in the absence of expressions of mixed variants (0%) (p < 0.01), with a major connection to the axial form of parasitic arthritis (Kendall-Tau index = 0.96; p < 0.001).
Parasitic arthritis due to Toxocara canis showed an overwhelming predominance of peripheral forms of joint syndrome (70.96%), with an insignificant number of axial (12.9%) and mixed forms (16.13%) (p < 0.01), with a connection to the peripheral form of parasitic arthritis (Kendall-Tau index = 0.93; p < 0.01).
The joint involvement associated with Giardia lamblia had a predominantly mixed joint syndrome (57.89%), a peripheral impairment – 36.84%, and axial – 5% with a statistically significant significance (p < 0.05), as well as a connection for the mixed-peripheral form of parasitic arthritis (Kendall-Tau index = 0.81; p < 0.05).
We have deduced from the results that the clinical expression of the joint syndrome correlates with the type of parasite, which dominates the disease, and this confirms the validity of etiology classification.
Discussions
The problem of parasitic infection in rheumatology has seen continuous increase of interest to various medical specialists – rheumatologists, infectious diseases, immunologists, biochemists, etc. Their interest is supported by the importance of parasitic agents in the development of rheumatic diseases, such as rheumatic syndromes associated with echinococcosis, toxoplasmosis, toxocariasis, etc. In all these cases, the parasite is starting a process of triggering of immunopathogenic processes in the „target” organs. The interest in this problem is also supported by the demonstrated ability of antiparasitic therapy to modulate the evolution of syndromes and rheumatic diseases. The effectiveness of antiparasitic therapy is well known in the invasions of parasites and other rheumatic diseases, in which the etiology of parasites is proven or suspected.
The severity of the inflammatory process is confirmed by significant values of the DAREA score (for giardiasis DAREA = 84.29±0.47, echinococcosis the score DAREA = 69.28±0.29 and toxocariasis – 59.55±0.51. The value p < 0.01 for parasitic arthritis by giardiasis vs. parasitic arthritis by toxocariasis, and p < 0.05 – for parasitic arthritis from echinococcosis, which is a sensitive indicator of disease activity . In addition, the VAS pain score presented significantly higher values in the group of patients with parasitic arthritis in Giardia lamblia (55.07±0.14) compared to Echinococcus (45.15±0.13) and Toxocara canis (37.04±0.19), with the deduced evidence showing statistical differences (p < 0.05).
The ASDAS-CRP score on aggregate confirmed the data of the DAREA score, but it drew them much more clearly and defined. Thus, according to the ASDAS-CRP score, the most severe form of parasitic arthritis by inflammatory syndrome was the parasitic arthritis of Giardia lamblia: manifested the highest values of the score, with the predominance of high activity of the disease (cumulatively 100% patients the score is >1.3), followed by parasitic arthritis in Echinococcus (cumulatively 90.73% patients the score is >1.3, p < 0.05) and the mildest variant of parasitic arthritis was presented the one with Toxocara canis (cumulatively 77.42% patients the score is >1.3, p < 0.01).
Given the existence of various forms of spinal damage in parasitic arthritis, we considered necessary to examine separately the values of the indices of vertebral damage BASDAI, BASFI and BASRI depending on the study group and the clinical form of parasitic arthritis. The study of these indices allowed us to confirm the heterogeneity of affecting the spine by chaotic migration of the values of the indices of statistical significance between groups.
When analyzing the BASDAI, BASFI and BASRI indices within the values of the group of patients with parasitic arthritis from echinococcosis, the giardiasis and toxocariasis groups, a statistically significant difference was determined only between the BASDAI index values (4.23±0.33; 5.07±0.28 vs 3.4±0.45; p < 0.005).
This confirms that damage to the spine is much more expressed in patients with parasitic arthritis of Giardia lamblia etiology and Echinococcus, than in those with parasitic arthritis associated with Toxocara canis. The data of the literature also confirms the direct link between the parasitic infestation and the spine involvement, which is considered a negative prognostic factor in the evolution of the systemic disorder [10, 11].
Conclusions
Parasitic arthritis is characterized by the diversity of clinical joint manifestations, which fall into three clinical variants: induced by infestation with Echinococcus, Toxocara canis and Giardia lamblia, among which giardiasis correlates with a more severe clinical course, followed by echinococcosis and toxocariasis. Despite a large number of tender and swollen joints that is also associated with a progression of radiological erosions, parasitic arthritis is characterized by a comparatively attenuated articular painful syndrome.
Abbreviations
ASDAS – Ankylosing Spondylitis Disease Activity Score; BASDAI – Bath Ankylosing Spondylitis Disease Activity Index; BASFI – Bath Ankylosing Spondylitis Functional Index; BASRI – Bath Ankylosing Spondylitis Radiology Index; CRP – C-reactive protein; DAREA – Disease Activity REactive Arthritis; FC – functional class; VAS – visual analogic scale.
Competing interests
None declared
Authors' contribution
Study conception and design: MG, LG. Data acquisition: MG, ER. Analysis and interpretation of data: MG, ER. Drafting of the manuscript: MG, ER. Significant manuscript review with significant intellectual involvement: LG. Approval of the „ready for print” version of the manuscript: LG, MG, ER.
Authors’ ORCID IDs
Maia Grosu – https://orcid.org/0000-0002-9390-9576
Liliana Groppa – https://orcid.org/0000-0002-3097-6181
Eugeniu Russu – https://orcid.org/0000-0001-8957-8471
References
Marquez J, Espinoza LR. Mycobacterial, brucellar, fungal and parasitic arthritis. In: Hochberg MC, editor. Rheumatology. 7th ed. Philadelphia: Elsevier; 2019. p. 943-54.
Painter JE, Gargano JW, Collier SA, Yoder JS; Centers for Disease Control and Prevention. Giardiasis surveillance - United States, 2011-2012. MMWR Suppl. 2015 May;64(3):15-25.
Painter JE, Collier SA, Gargano JW. Association between Giardia and arthritis or joint pain a large health insurance cohort: could it be reactive arthritis? Epidemiol Infect. 2017 Feb;145(3):471-477. doi: 10.1017/S0950268816002120.
Lewis JM, Clifford S, Nsutebu E. Toxoplasmosis in immunosuppressed patients. Rheumatology (Oxford). 2015 Nov;54(11):1939-40. doi:10.1093/rheumatology/kev115.
Hosseininejad Z, Sharif M, Sarvi S, Amouei A, Hosseini S.A, et al. Toxoplasmosis seroprevalence in rheumatoid arthritis patients: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018 Jun 5;12(6):e0006545. doi: 10.1371/journal.pntd.0006545.
Alim B, Centinel S, Servi MA, Bostanci F, Bingol MO. The case of reactive arthritis secondary to Echinococcus infestation. Case Rep Rheumatol. 2017;3293060 https://doi.org/10.1155/2017/3293060.
Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004 Jan;17(1):107-135. doi: 10.1128/CMR.17.1.107-135.2004.
Alishani M, Sherifi K, Rexhepi A, Hamidi A, Armua-Fernandez M, et al. The impact of socio-cultural factors on transmission of Taenia spp. and Echinoccus granulosus in Kosovo. Parasitology. 2017 Nov;144(13):1736-1742. doi: 10.1017/S0031182017000750.
Dsilva G, Kulkarni V, Aher S. An uncommon manifestation of a common disease. Ann Parasitol. 2017;63(4):357-360. doi: 10.17420/ap6304.124.
Hjollo T, Bratland E, Steinsland H, Radunovic M, Langeland N. Longitudinal cohort study of serum antibody responses towards Giardia lamblia variant-specific surface proteins in a non-endemic area. Exp Parasitol. 2018 Aug;191:66-72. doi: 10.1016/j.exppara.2018.06.005.
McSorley HJ, Maizels MR. Helminth infections and host immune regulation. Clin Microbiol Rev. 2012 Oct;25(4):585-608. doi: 10.1128/CMR.05040-11.