Introduction
Marginal zone lymphomas (MZLs) are a group of slow-growing B-cell tumours, the second most common non-Hodgkin lymphomas (NHL lymphomas). There are three types of marginal zone lymphomas: the extranodal MZL (EMZL) of mucosa-associated lymphoid tissue (MALT or gastric GALT), the splenic MZL, and the nodal MZL [1]. The gastrointestinal tract is the most affected site, with the involvement of the stomach in 2/3 of cases [2].
MALT lymphoma, or extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue, accounts for 50% of gastric lymphomas and represents the most common type of extranodal non-Hodgkin lymphoma. It can develop anywhere in the gastrointestinal tract, but most cases start in the stomach. Frequent non-gastric sites include orbit, lungs, and skin [3].
Tumour development is strongly associated with Helicobacter pylori (H. pylori) Cytotoxin-associated gene A strain-positive infection, according to a specific strain-host-organ process, with generally benign evolution. Eradication of H. pylori infection ensures complete remission of the gastric tumour in 75% of cases. Other recognised risk factors are hepatitis B virus (HVB), human immunodeficiency virus (HIV), and Epstein-Barr virus (EBV) infections [3-5].
In daily medical practice, clinical suspicion of gastric MALT lymphoma is practically absent, with patients presenting a wide range of vague, unspecific symptoms from dyspepsia to the presence of alarm signs. The appearance on white light endoscopy picture is not specific, with mucosal changes imitating different clinical entities, such as gastritis, peptic ulcer, polyps, early or advanced gastric cancer and subepithelial lesions, so this diagnosis is often overlooked [6].
In the following report, a unique case is presented of an advanced MALT lymphoma patient, unresponsive to H. pylori eradication treatment, with HBV hepatitis and an atypical onset of upper digestive bleeding.
Clinical case presentation
A 52-year-old woman presented in March 2023 to Institute of Oncology in Chisinau with repeated episodes of melena over the last month, progressive asthenic syndrome, and insignificant weight loss over the last half a year.
She has suffered from gastrointestinal tract disease since 2018, being admitted that year to the emergency room with syncope and severe anaemia. At that time, the patient was diagnosed with gastric ulcer Forrest IIb. of H. pylori origin. The biopsy of the lesion did not reveal the presence of tumour cells. For differential diagnosis and to exclude gastric neoplasia with submucosal extension, an abdominal computed tomography (CT) scan was performed, revealing an unexplained gastric and retro-pancreatic lymphadenopathy. The complicated peptic ulcer was treated conservatively, and the H. pylori infection was subsequently eradicated with bismuth subsalicylate quadruple therapy. From 2019 to 2022, due to persistent epigastric discomfort and lymphadenopathy, the patient underwent multiple upper endoscopies with histological examination of gastric biopsies and abdominal CT, but without the identifying a specific pathology.
The anamnesis revealed a history of untreated hypertension and HBeAg-negative chronic HBV infection. There was no history of smoking or alcohol consumption, and the patient denied a family history of upper gastrointestinal bleeding, oesophageal, or gastric cancer.
On physical examination, the patient was hemodynamically stable with blood pressure of 120/70 mmHg, heart rate of 70 bpm, and an oxygen saturation of 96% on room air. No stigmata of chronic liver disease were observed. The abdomen was soft, tender to palpation in the epigastrium, and supple, with normal bowel sounds. Rectal examination showed no evidence of melena on examination. Laboratory investigations revealed no significant changes; general blood analysis, biochemical examination, and tumour markers were within reference values.
Upper gastrointestinal endoscopy revealed an area of hard submucous infiltration with deep ulcerative destruction, about 20 mm in size, covered with yellowish fibrin, adjacent foveolar hyperplasia with a relatively regular pattern, erythema and petechiae, localized in the proximal area of the gastric body, on the lesser curvature and anterior wall. On palpation, the area of infiltration was excessively hard. Multiple biopsy specimens were taken from the lesion and the stomach. Histologic examination revealed a severely expressed lymphocytic inflammatory infiltrate. The Rapid Urease Test was negative. Endoscopic images of the gastric mucosa can be visualized in Figure 1.
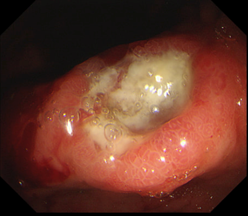 | 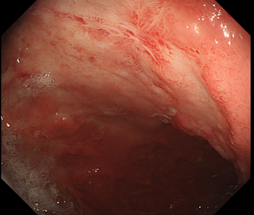 |
Fig. 1 Endoscopic examination of the gastric mucosa a. Ulcerative lesion; adjacent mucosal infiltrate; foveolar hyperplasia. b. Erythema and petechiae adjacent to the ulcerative lesion. | |
Non-Hodgkin's lymphoma or proximal gastric cancer was suspected. An abdominal computed tomography with contrast, performed 1 month previously, was used to stage the tumor, which showed perigastric, peripancreatic, and intestinal lymphadenopathy up to 1.9 cm in size.
For confirmation of the diagnosis, the patient underwent median laparotomy with total gastrectomy and Roux-en-Y loop reconstruction following the double-tract method and lymph node dissection. During surgery, a firm, infiltrative tumor of about 8x10 cm was determined. Along the common hepatic artery - lymphadenopathy up to 3.5 cm. Histopathological examination of the resection specimen revealed an extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue with primary involvement of the stomach characterized by proliferation of small lymphoid cells of B-cell morphology. Further immunophenotyping tests were performed with the following results: tumor cells diffusely positive for CD45(+), CD20(+), BCL2(+) and negative for BLC6(-), CD5(-), CD23(-), Cyclin D1(-), with transmural involvement of the gastric wall. Ten lymph nodes along the lesser curvature were investigated. In only one nodule tumor infiltration of lymphoid tissue was found. A diagnosis of MALT lymphoma, Ann-Arbor stage IVA, with involvement of the stomach, perigastric, peripancreatic, and intestinal lymph nodes was established.
The postoperative course was uncomplicated, the patient was discharged 11 days after gastrectomy. Because of the risk of lymphomatous lesions at other mucosal sites and the advanced stage of the disease, adjuvant polychemotherapy (PChT) was administered, consisting of 8 courses of PChT: one COP session (cyclophosphamide, vincristine, prednisone) visit, two R-COP sessions (rituximab, cyclophosphamide, vincristine, prednisone) and five R-CHOP sessions (rituximab, doxorubicin hydrochloride, cyclophosphamide, vincristine, prednisone).
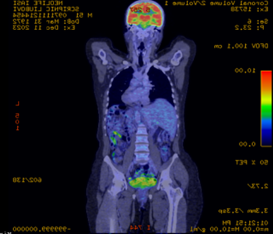
The patient continues to be monitored clinically and by imaging. 18F-Fluorodeoxyglucose Positron Emission Tomography-computed Tomography (FDG PET-CT) was performed 2 months (January 2024) after completion of induction chemotherapy. According to the classification of the glucose metabolism, it showed a reduced tracer uptake at the right axillary, lateral aortic and left mesenteric lymph nodes, corresponding to a Deauville score 2, considered a complete metabolic response (Figure 2).
Discussion
A clinical case was presented of advanced-stage MALT lymphoma with an unusual onset of upper gastrointestinal bleeding. The comprehensive diagnostic workup spanned over 5 years, entailing the collaborative efforts of gastroenterologists, oncologists, radiologists, and hematologists.
The median age of onset of MALT lymphoma is 57 years, similar to the patient presented, with a male/female ratio of 1.27:1. The clinical presentation is diverse and often asymptomatic, with the presence of warning signs, such as weight loss, hemorrhage, fever in only 42.1% of cases. Only a few cases of MALT lymphoma are characterized by the presence of peptic ulcer, with dyspepsia and pain syndrome. Disease onset with HDS as in this patient is not a usual manifestation and has been reported in only 15.6% of cases in some retrospective studies [7, 8]. It is the clinician's responsibility to consider gastric lymphoma as the potential cause of such symptoms, particularly in patients with persistent chronic ulcers and recurrent bleeding despite targeted treatment. The patient's case serves as an instructive example in this regard.
| 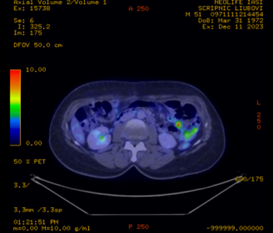 |
Fig. 2 FDG PET-CT reduced accumulation of 18-fluorodeoxyglucose, complete metabolic response. a. sagittal section, b. cross-section | |
Endoscopic features in patients with gastric MALT lymphoma are varied, categorized as ulcerative (34- 69%), polypoid (26- 35%), diffuse infiltrative (15- 40%) and other types. The use of narrow-band imaging (NBI) during endoscopy may be helpful. Another endoscopic characteristic of MALT lymphoma is the presence of abnormally large capillaries “tree trunk-like, with long empty branches”. With the advent of endoscopic microscopy, the presence of intraglandular aggregation of cellular components and small-sized nuclei in gastric lymphoma and, the presence of lymphoepithelial lesions were detected, but additional studies are needed [9, 10]. On upper gastrointestinal endoscopy performed on this patient, foveolar hyperplasia with a regular vascular pattern was determined.
However, the primary method for diagnosing the disease is histologic examination. It is important to mention the need to perform multiple biopsies, at least 10 biopsies in and outside the lesion to increase the chances of detection and to exclude the association of other precancerous conditions [11].
Given the association of MALT lymphoma with H. pylori infection, the ideal treatment option in patients with gastric lymphoma of low malignancy is its eradication, which results in complete remission in about 78% of cases. MALT lymphoma is currently the only cancer that can be treated with antibiotics. Resistance to treatment is associated with advanced stage III/IV disease, absence of infection, proximal localization of the tumour in the stomach, and the presence of infiltrative endoscopic type [3, 9, 12].
In this patient, treatment for H. pylori infection was performed incidentally in the context of managing the first episode of upper gastrointestinal bleeding, which was considered to be due to a complicated gastric ulcer. Eradication of the infection was confirmed with a negative H. pylori antigen test in the stool sample. However antibacterial treatment did not demonstrate efficacy in tumor regression, reflecting the uniqueness of the case. It can only be speculated that the lack of results was motivated by the advanced stage of the disease as early as 2018, tumour localization in the proximal 1/3 of the gastric body and infiltrative endoscopic form.
The second-line treatment for gastric MALT lymphoma, following the failure of eradication treatment and in cases of complications such as upper gastrointestinal bleeding, is total resection of the stomach. In some limited cases, radiotherapy may be recommended, while chemotherapy may be used for patients with extensive and aggressive forms of the condition. The chemotherapy protocol used in this patient, namely R-CHOP and R-COP, is considered effective and well-tolerated in clinical trials as well [12, 13].
FDG PET-CT is recommended for monitoring treatment efficacy in aggressive MALT lymphoma. The Deauville scale is a simple scoring system based on visual interpretation of FDG uptake with 2 reference points: mediastinum and liver. A Deauville score of 2, as in the patient in question, is considered to be a mild uptake associated with a complete metabolic response. This denotes the absence of a malignant process in the lymph nodes analysed and a low risk of recurrence. However, the prognostic value of FDG PET-CT is uncertain and requires further research [14].
Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue are considered exceptionally indolent tumours. Most patients can expect their longevity to be unaffected by disease-associated complications. However, MALT lymphoma may have a heterogeneous course of progression. In a retrospective study with analysis of 593 patients with MALT lymphoma, the 5- and 10-year mortality rates were 2.8% and 4.3%, the identified independent risk factors were age over 70 years and advanced stage of disease [15].
The prognosis of this patient is positive, given the PET-CT findings and lack of other risk factors, but careful monitoring remains necessary.
Conclusions
Gastric MALT lymphoma is a rare condition that, in the early stages of the disease, can be managed solely by antibiotic therapy. Defined by a multitude of nonspecific symptoms andoften asymptomatic, this pathology can also be termed a “big masquerader” that requires maximum vigilance in making the diagnosis. A thorough endoscopic examination, with an adequate number of biopsies, along with the use of NBI technology, carried out by a skilled specialist is essential for both detection and subsequent monitoring. Although it generally follows a favourable clinical course, it requires a multidisciplinary approach and chemotherapeutic treatment in advanced stages.
Competing interests
None declared.
Authors’ contribution
ET conceived the study, participated in the study design and reviewed the article. COS participated in the study design and drafted the manuscript. MS made a substantial contribution to the data acquisition. AT revised critically the article, ensuring accuracy and validation. LA, MC conducted the investigation process and evidence collection. All the authors reviewed the work critically and approved the final version of the manuscript.
Informed consent for publication
Obtained.
Funding
None.
Authors’ ORCID IDs
Eugen Tcaciuc – https://orcid.org/0000-0002-2362-1788
Cătălina Olaru-Stăvilă – https://orcid.org/0009-0005-3524-427X
Angela Tcaciuc – https://orcid.org/0000-0002-5530-0946
Lilian Antoci – https://orcid.org/0000-0001-6908-129X
Mircea Cernat – https://orcid.org/0000-0002-1449-4342
Margareta Surlari – https://orcid.org/0009-0004-8549-2047
References
Robu M, Corcimaru I, Musteață L, et al.; [Ministry of Health of the Republic of Moldova]. Limfomul non-Hodgkin la adult: Protocol clinic naţional (PCN-46) [Non-Hodgkin lymphoma in adults: National clinical protocol] [Internet]. Chişinău: The Ministry; 2019 [cited 2024 Sep 2]. Romanian. Available from: https://cemf.md/wp-content/uploads/2023/07/PCN-46-Limfom-Hodgkin-la-adu….
Alvarez-Lesmes J, Chapman JR, Cassidy D, Zhou Y, Garcia-Buitrago M, Montgomery EA, et al. Gastrointestinal tract lymphomas. Arch Pathol Lab Med. 2021 Apr 9;145(12):1585-96. doi: 10.5858/arpa.2020-0661-ra.
Zullo A, Hassan C, Ridola L, Repici A, Manta R, Andriani A. Gastric MALT lymphoma: old and new insights. Ann Gastroenterol. 2014;27(1):27-33.
Du MQ, Isaccson PG. Gastric MALT lymphoma: from aetiology to treatment. Lancet Oncol. 2002 Feb;3(2):97-104. doi: 10.1016/s1470-2045(02)00651-4.
Filip VP, Cuciureanu D, Diaconu SL, Vladareanu AM, Pop SC. MALT lymphoma: epidemiology, clinical diagnosis and treatment. J Med Life. 2018 Jul-Sep;11(3):187-193. doi: 10.25122/jml-2018-0035.
Hassegawa RT, Ogawa EKM, El Ibrahim R, Venco FE, Maruta LM. Pre-malignant signs of gastric MALT lymphoma. Autops Case Rep. 2019 Dec 13;10(1):e2019130. doi: 10.4322/acr.2019.130.
Zullo A, Hassan C, Cristofari F, Perri F, Morini S. Gastric low-grade mucosal-associated lymphoid tissue-lymphoma: Helicobacter pylori and beyond. World J Gastrointest Oncol. 2010 Apr 15;2(4):181-6. doi: 10.4251/wjgo.v2.i4.181.
Song Y, Jiang K, Su S, Wang B, Chen G. Clinical manifestations and epigenetic mechanisms of gastric mucosa associated lymphoid tissue lymphoma and long-term follow-up following Helicobacter pylori eradication. Exp Ther Med. 2018 Jan;15(1):553-561. doi: 10.3892/etm.2017.5413.
Nakamura S, Hojo M. Diagnosis and treatment for gastric mucosa-associated lymphoid tissue (MALT) lymphoma. J Clin Med. 2022 Dec 23;12(1):120. doi: 10.3390/jcm12010120.
Matysiak-Budnik T, Priadko K, Bossard C, Chapelle N, Ruskoné-Fourmestraux A. Clinical management of patients with gastric MALT lymphoma: a gastroenterologist's point of view. Cancers (Basel). 2023 Jul 27;15(15):3811. doi: 10.3390/cancers15153811.
Dreyling M, Thieblemont C, Gallamini A, Arcaini L, Campo E, Hermine O, et al. ESMO Consensus conferences: guidelines on malignant lymphoma. part 2: marginal zone lymphoma, mantle cell lymphoma, peripheral T-cell lymphoma. Ann Oncol. 2013 Apr;24(4):857-77. doi: 10.1093/annonc/mds643.
Sugizaki K, Tari A, Kitadai Y, Oda I, Nakamura S, Yoshino T, Sugiyama T. Anti-Helicobacter pylori therapy in localized gastric mucosa-associated lymphoid tissue lymphoma: a prospective, nationwide, multicenter study in Japan. Helicobacter. 2018 Apr;23(2):e12474. doi: 10.1111/hel.12474.
Thieblemont C, Zucca E. Clinical aspects and therapy of gastrointestinal MALT lymphoma. Best Pract Res Clin Haematol. 2017 Mar-Jun;30(1-2):109-117. doi: 10.1016/j.beha.2017.01.002.
Qi S, Liu X, Noy A, Lee J, Teckie S, Hajj C, et al. Predictors of survival in patients with MALT lymphoma: a retrospective, case-control study. Blood Adv. 2023 Apr 25;7(8):1496-1506. doi: 10.1182/bloodadvances.2022007772.
Ceriani L, Meignan M. Present role and future perspective of PET-CT in marginal zone lymphoma. Ann Lymphoma. 2020;4:13-13. doi: 10.21037/aol-20-13.